

Background: Patients (pts) with non-radiographic axial spondyloarthritis (nr-axSpA) who fail nonsteroidal anti-inflammatory drug (NSAID) therapy are candidates for tumor necrosis factor inhibitor (TNFi) therapy if they have objective signs of inflammation. Baseline predictors of response to TNFi therapy, including remission, may aid clinical management.
Objectives: Describe baseline predictors of remission in nr-axSpA at wk 12 of open-label adalimumab (ADA) therapy in the ABILITY-3 study.
Methods: ABILITY-3 enrolled adult pts with nr-axSpA (fulfilling Assessment of SpondyloArthritis international Society [ASAS] criteria but not modified New York criteria) with moderately to severely active disease at screening and baseline, objective evidence of inflammation in the sacroiliac (SI) joints or spine on magnetic resonance imaging (MRI) or elevated high-sensitivity C-reactive protein (hs-CRP; defined as > upper limit of normal for the lab) at screening, and an inadequate response to ≥2 NSAIDs. Eligible pts received ADA 40 mg every other week during a 28-wk open-label lead-in period. Clinical remission was defined as Ankylosing Spondylitis Disease Activity Score inactive disease (ASDAS ID; score <1.3) or ASAS partial remission (score <2/10 in each of the 4 ASAS domains). Stepwise logistic regression was used to identify potential baseline predictors of remission at wk 12.
Results: 673 pts were enrolled (Table). Lower disease activity, increased inflammation (morning stiffness), less functional limitation (BASFI), younger age, presence of human leukocyte antigen-B27 (HLA-B27), and positive MRI of the SI joints were the strongest predictors of ASDAS ID; higher hs-CRP levels, younger age, lower functional status (HAQ-S), presence of HLA-B27, and positive MRI of the SI joints were the strongest predictors of ASAS PR (Figure).
Table 1. Baseline Characteristics BASFI, Bath Ankylosing Spondylitis Functional Index; HAQ-S, Health Assessment Questionnaire modified for the Spondyloarthropathies. *Mean of Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) questions 5 and 6 (scale, 0–10).
Mean ± SD or n (%) ASDAS ID ASAS PR Responders Non-responders Responders Non-responders (n=211) (n=389) (n=133) (n=470) Age, y 33.6±9.7 38.9±11.4 31.8±8.7 38.5±11.2 Diagnosis duration, y 1.7±2.9 1.8±3.6 1.7±2.9 1.7±3.2 Symptom duration, y 6.1±6.2 8.2±8.1 5.3±5.7 8.0±7.8 HLA-B27 positive 183 (87) 274 (70) 119 (89) 340 (72) ASDAS 3.4±0.8 3.7±0.8 3.7±0.9 3.6±0.8 Inflammation* 6.9±1.8 7.0±2.0 6.9±1.9 7.0±1.9 Patient global assessment of pain 7.0±1.7 7.7±1.5 7.1±1.9 7.5±1.6 hs-CRP 9.0±13.1 10.9±16.8 15.5±21.3 8.8±13.1 BASFI 4.6±2.2 5.7±2.2 4.9±2.4 5.4±2.2 HAQ-S 1.9±0.5 2.2±0.5 1.9±0.6 2.1±0.5 MRI SI joints positive 165 (78) 262 (67) 102 (77) 328 (70) MRI spine positive 59 (28) 114 (29) 47 (35) 126 (27)
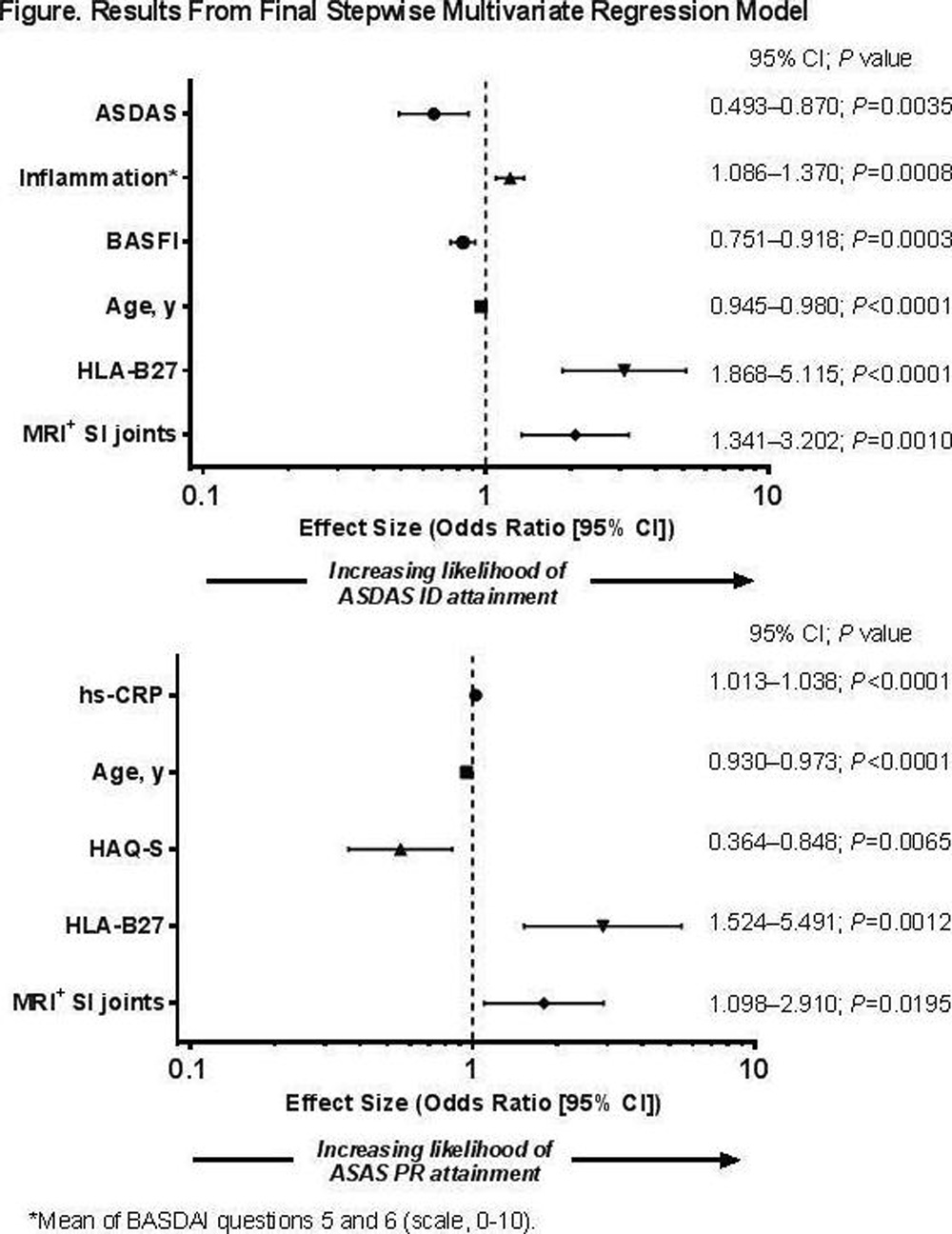
Conclusions: In ABILITY-3, younger age, better functional status, presence of HLA-B27, and positive MRI of the SI joints consistently predicted clinical remission at wk 12 of open-label ADA treatment in pts with nr-axSpA.
Acknowledgements: AbbVie funded the study and approved the abstract for submission. Medical writing support was provided by Maria Hovenden, PhD, of Complete Publication Solutions, LLC (North Wales, PA) and was funded by AbbVie.
Disclosure of Interest: J. Sieper Grant/research support from: AbbVie, Merck, Pfizer, and UCB, Consultant for: AbbVie, Merck, Pfizer, and UCB, Speakers bureau: AbbVie, Merck, Pfizer, and UCB, R. Landewé Grant/research support from: Abbott, Amgen, Centocor, Novartis, Pfizer, Roche, Schering-Plough, UCB, and Wyeth, Consultant for: Abbott/AbbVie, Ablynx, Amgen, Astra-Zeneca, Bristol Myers Squibb, Celgene, Janssen (formerly Centocor), Galapagos, GlaxoSmithKline, Novartis, Novo-Nordisk, Merck, Pfizer, Roche, Schering-Plough, TiGenix, UCB, and Wyeth, Speakers bureau: Abbott/AbbVie, Amgen, Bristol Myers Squibb, Janssen (formerly Centocor), Merck, Pfizer, Roche, Schering-Plough, UCB, Wyeth; he is director of Rheumatology Consultancy BV, a registered Dutch company., M. Magrey Grant/research support from: Amgen, AbbVie, and UCB Pharma, Consultant for: UCB and Janssen, J. Anderson Employee of: AbbVie, S. Zhong Employee of: AbbVie, X. Wang Employee of: AbbVie, A. Lertratanakul Employee of: AbbVie
DOI: 10.1136/annrheumdis-2017-eular.6467