

Background: A phase characterised by the presence of specific autoantibodies and arthralgia’s in the absence of clinically evident synovial inflammation often precedes the onset of rheumatoid arthritis (RA). However, only a subset of these RA-risk individuals will develop active disease in the short term1. Recent findings show that dominant B-cell receptor (BCR) clones in peripheral blood can accurately predict imminent onset of arthritis in these RA-risk individuals.2
Objectives: To validate the predictive role of BCR clones in peripheral blood in RA-risk individuals in a larger cohort.
Methods: The BCR repertoire in peripheral blood was analysed using next-generation BCR sequencing in a prospective cohort study of 129 RA-risk individuals from Reade. Like earlier, BCR clones expanded beyond 0.5% of the total repertoire were labelled highly expanded clones (HECs), shortly referred to as dominant BCR clones, and individuals were labelled BCR-positive if peripheral blood at study baseline showed ≥5 dominant BCR clones.
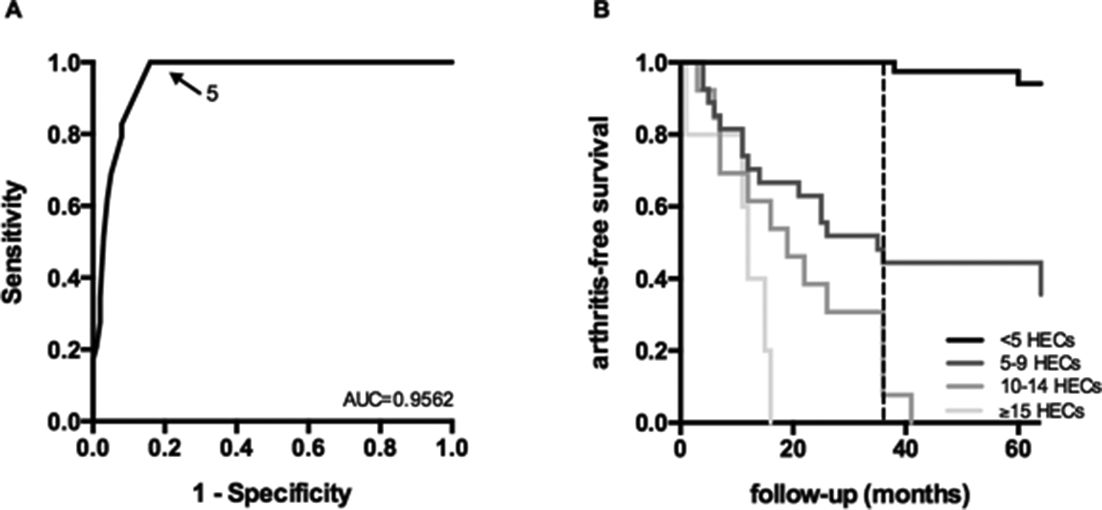
Results: We observed that the number of dominant BCR clones was increased in RA-risk individuals who developed arthritis within 3 years, compared to RA-risk individuals who did not 10.6±5.3 vs 2.2±2.8 (mean ±SD; p<0.0001). When creating a ROC curve we could replicate that the most optimal cut-off for this test is at ≥5 dominant BCR clones in the peripheral blood (figure 1A), dividing the cohort in 45 BCR-positive individuals and 84 BCR-negative individuals. None of the BCR-clone negative individuals developed arthritis within 36 months. Within the total follow-up of 104 months only 8% of the BCR-clone negative individuals developed arthritis compared to 76% of the BCR-clone positive individuals, resulting in a relative risk of 9.1 (95% CI: 4.4 to 18.8, p<0.0001).
To test whether a higher number of dominant BCR clones correlates with higher risk of arthritis BCR-clone positive individuals were subdivided into three groups: 5–9 HECs (n=27), 10–14 HECs (n=13) and ≥15 HECs (n=5). The Kaplan-Meier curve for all groups is shown in figure 1B (logrank test between BCR-clone negative group and positive subgroups: p<0.0001). Having 10 or more HECs corresponded with a positive predictive value of 83% and a negative predictive value of 87% within 3 years. The BCR clonality test clearly added to existing indices of RA risk in RA-risk individuals (data not shown).
Abstract OP0204 – Figure 1 Predictive value of dominant BCR clones (A) reciver operating characteristic (ROC) curves for the number of dominant clones, in at-risk individuals (dotted line at 36 months)
Conclusions: In this external validation cohort we could replicate the fact that dominant BCR clones in peripheral blood predict imminent onset of clinical symptoms of RA in seropositive arthralgia patients with high accuracy. Furthermore, a highly significant association correlating a higher number of dominant BCR clones with higher risk was shown. We hope these results will support evaluation of early interventions that prevent onset of arthritis.
References
Acknowledgements: Intellectual property PCT/EP2017/067047. N de Vries, PP Tak, ME Doorenspleet, PL Klarenbeek. ‘METHOD FOR DETERMINING THE RISK OF DEVELOPING ARTHRITIS’.
Disclosure of Interest: A. Musters: None declared, M. van Beers-Tas: None declared, M. Doorenspleet: None declared, P. Klarenbeek: None declared, B. van Schaik: None declared, A. van Kampen: None declared, F. Baas: None declared, D. van Schaardenburg: None declared, N. de Vries Grant/research support from: IMI (ABIRISK, BeTheCure), CTMM (TRACER), LSH (MODIRA), Pfizer, Roche, Janssen, GSK, Consultant for: UCB, MSD
DOI: 10.1136/annrheumdis-2018-eular.3471