

Background: Giant cell arteritis (GCA) is the most common form of systemic vasculitis; the pathogenesis is unclear. Current evidence suggests both the TH1 and TH17 pathways are important but the proximal initiators and effector cytokines are unknown. IL-12 and IL-23 secreted by dendritic cells are hypothesised as stimulators of these pathways. We have previously reported the efficacy of IL-12/23 blockade with ustekinumab in refractory GCA in a prospective clinical trial.
Objectives: To assess the role of IL-12 and IL-23 in GCA pathogenesis.
Methods: IL-12 and IL-23 were quantified by immunohistochemistry in temporal artery (TA) biopsies. TA explant, peripheral blood mononuclear cell (PBMC), and myofibroblast outgrowth culture models were established from patients with GCA and disease controls. PBMCs and TA explants were cultured for 24 hours in the presence or absence of IL-23 (10 ng/ml) or IL-12 (50 ng/ml). Gene expression was quantified by Real-time PCR and cytokine secretion by ELISA. Myofibroblast outgrowths were assessed following 28 days culture and quantified by counting the number of outgrowths/high-power field (hpf).
Results: Immunohistochemistry demonstrated IL-12p35 and IL-23p19 in inflammatory cells in TA biopsies (n=33). IL-12p35 and IL-23p19 were only detected in positive TA biopsies. IL-12p35 was increased in those with cranial ischaemic complications (p=0.026) and those with large vessel vasculitis (p=0.006). IL-23p19 was increased in those with two or more relapses (p=0.007). In cultured PBMCs, IL-12 stimulation increased IL-6 (n=17, p=0.009), IL-22 (n=16, p=0.003), and IFN-γ (n=14, p=0.0001) secretion and decreased IL-8 (n=15, p=0.0006) secretion, while IL-23 stimulation increased IL-6 (n=40, p=0.029), IL-22 (n=16, p=0.001), IL-17A (n=16, p=0.0003) and IL-17F (n=9, p=0.012) secretion. In the TA explant culture model, IL-23 stimulation increased gene expression of IL-8 (n=13, p=0.0001) and CCL-20 (n=9, 0.027) and protein expression of IL-6 (n=61, p=0.002) and IL-8 (n=60, p=0.004), IL-12 stimulation (n=14) had no effect; however, IFN-γ and IL-17A were not detectable in this model. IL-12 (n=20, p=0.0005) and IL-23 (n=33, p<0.0001) stimulation increased the quantity of myofibroblast outgrowths from TA biopsies. In all experiments there were no significant differences between biopsy positive GCA, biopsy negative GCA, and disease controls.
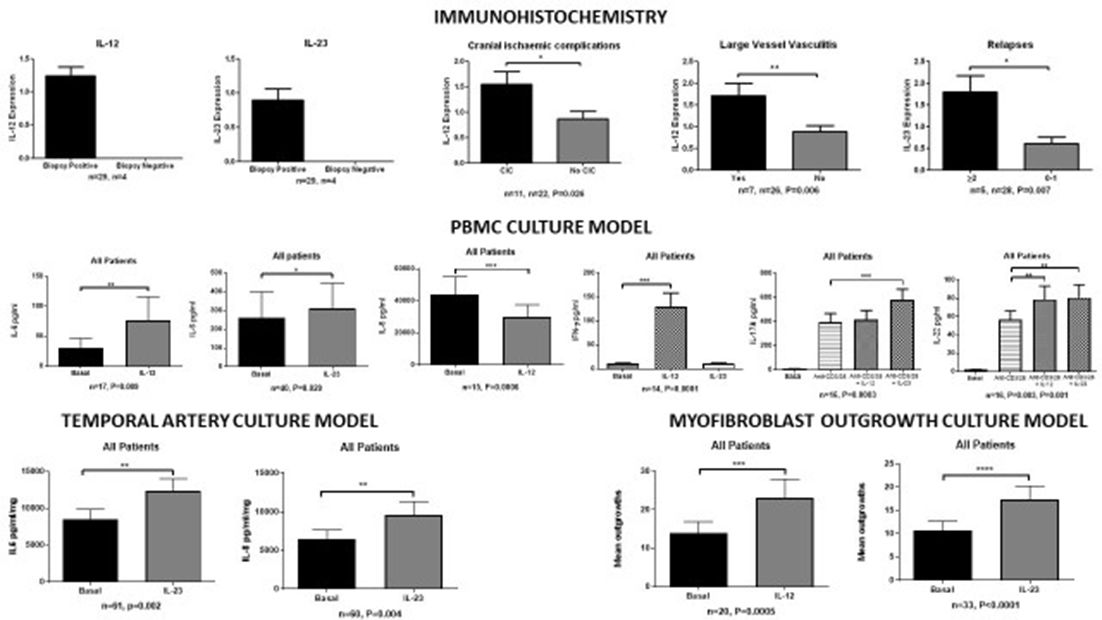
Conclusions: IL-12 and IL-23 play central and distinct roles in stimulating inflammatory and proliferative pathways in GCA. Our results were consistent in patients with biopsy positive and negative GCA, and in disease controls, suggesting that IL-12 and IL-23 play proximal roles in inducing these pathways.
Disclosure of Interest: None declared
DOI: 10.1136/annrheumdis-2018-eular.2850