

Background: Superior rates of sustained glucocorticoid (GC)–free remission were shown in patients with giant cell arteritis (GCA) treated with weekly or every-other-week (wk) subcutaneous tocilizumab (TCZ) 162 mg +26 wk GC taper for 52 wks compared with placebo +26 wk or 52-wk GC taper (PBO +26 or PBO +52) in the GiACTA trial. Statistically significant improvements in SF-36 Physical Component Summary (PCS) scores were reported for weekly TCZ vs PBO +52 and in patient-reported global assessment of disease activity for both TCZ groups vs both PBO groups.1
Objectives: To report further analysis of patient-reported outcomes (PROs) in GiACTA.
Methods: Analyses of SF-36 PCS and Mental Component Summary (MCS), SF-36 domains, and Functional Assessment of Chronic Illness Therapy (FACIT)–fatigue compared patients treated with weekly TCZ (n=100) vs PBO +26 (n=50; not shown) or PBO +52 (n=51) for 52 wks based on reported data, including all responders as well as patients with post-escape data following flare.
Results: Improvements in SF-36 PCS and MCS scores, 6 of 8 SF-36 domains, and FACIT–Fatigue at wk 52 were significantly greater with weekly TCZ vs PBO +52 (p<0.01) (table 1, figure 1). At wk 52, mean scores met or exceeded age/gender (A/G)–matched normative scores in the weekly TCZ group; higher proportions of patients reported scores exceeding A/G norms in SF-36 PCS and MCS, all SF-36 domains, and FACIT-Fatigue (Table) compared with PBO groups. The median cumulative prednisone dose over 52 wks was lower with weekly .TCZ (18620 mg) vs PBO +26 (3296.0 mg) or PBO +52 (3817.5 mg) (p<0.01).
Weekly TCZ+26 n=100 |
PBO+52 n=51 |
|||||
|---|---|---|---|---|---|---|
Baseline |
Wk 52 |
LSM Δ |
Baseline |
Wk 52 |
LSM Δ |
|
PROs (A/G norms) |
||||||
PtGA |
43.61 |
24.36 |
–17.14 |
47.78 |
35.44 |
–7.56 |
FACIT-Fatigue (40.0) |
36.05 (43.4) |
42.08 (73.8) |
5.30a |
31.42 (32.7) |
32.62 (35.6) |
–0.42 |
SF-36 PCS (50.0) |
43.10 (23.7) |
47.75 (43.5) |
4.18a |
41.12 (20.4) |
41.24 (22.2) |
–0.40 |
SF-36 MCS (50.0) |
42.77 (33.0) |
51.64 (60.0) |
8.10a |
40.45 (34.7) |
44.86 (40.0) |
1.89 |
SF-36 Domains (A/G norms) |
||||||
Physical function (67.56) |
69.10 (60.0) |
78.28 (78.8) |
6.83 |
59.40 (42.0) |
65.44 (55.6) |
2.68 |
Role physical (69.44) |
49.56 (25.0) |
73.75 (56.5) |
20.64a |
45.38 (28.0) |
53.89 (33.3) |
4.46 |
Bodily pain (64.52) |
61.93 (41.0) |
73.25 (65.9) |
10.89a |
55.67 (34.7) |
56.27 (31.1) |
–2.87 |
General health (66.49) |
55.00 (25.8) |
65.81 (57.6) |
9.06a |
55.69 (36.0) |
52.29 (22.0) |
–4.05 |
Vitality (58.65) |
50.19 (33.3) |
66.13 (68.2) |
15.69a |
42.38 (28.0) |
49.17 (33.3) |
3.53 |
Social function (81.49) |
64.25 (29.0) |
84.71 (63.5) |
17.35a |
63.00 (40.0) |
67.50 40.0 |
2.34 |
Role emotional (82.08) |
66.38 (43.0) |
82.45 (62.4) |
13.37 |
60.33 (36.0) |
69.63 (46.7) |
3.53 |
Mental health (77.16) |
64.04 (32.3) |
77.94 (52.9) |
12.54a |
59.10 (24.0) |
66.33 (31.1) |
3.13 |
Abstract SAT0550 – Figure 1 SF-36 Domains at BL and Week 52. **p<0.01 vs PBO+52. A/G, age/gender; BL, baseline.
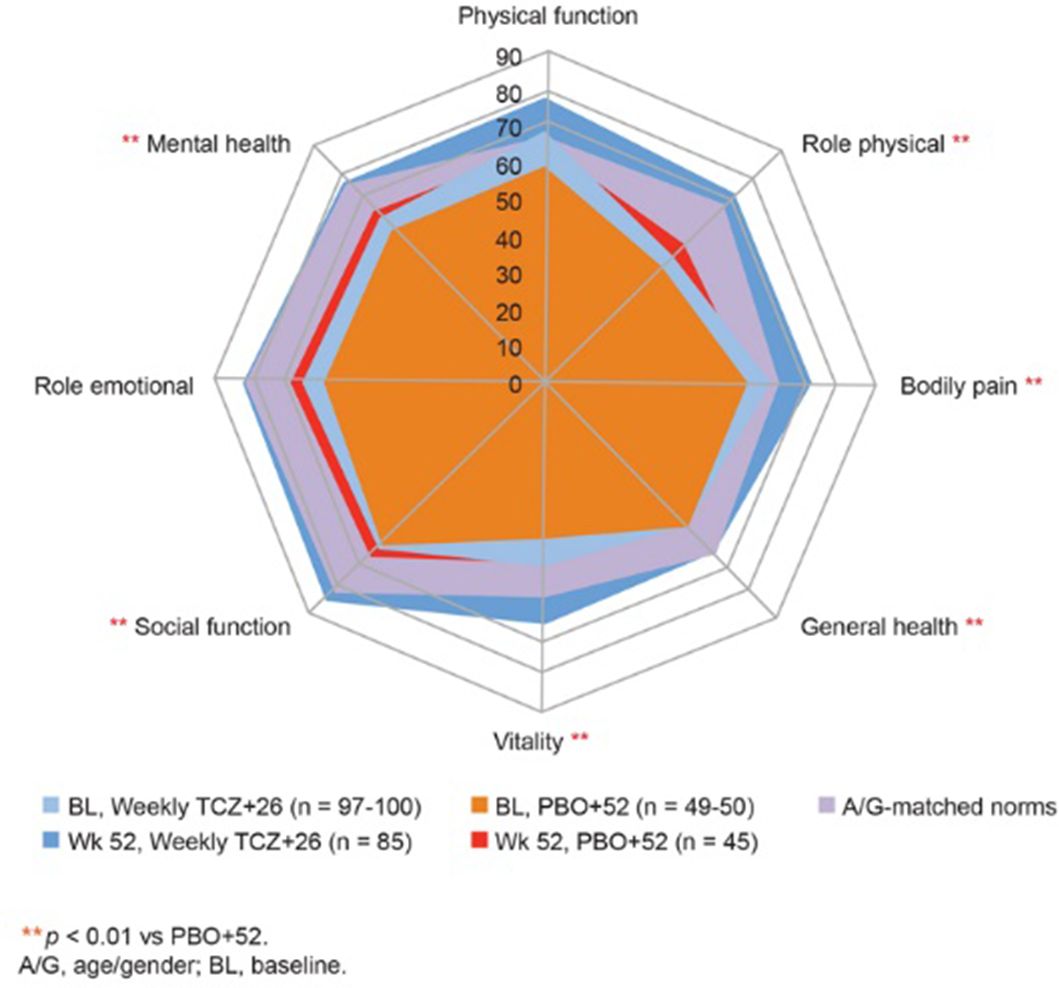
Conclusions: Patients with GCA treated with weekly TCZ 162 mg and a 26-wk GC taper reported statistically significantly greater improvements in health-related quality of life and fatigue that exceeded normative values compared with those receiving 52-wk GC taper alone, in part ascribed to lower steroid doses.
Reference:
Disclosure of Interest: V. Strand Consultant for: AbbVie, Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Celltrion, CORRONA, Crescendo, EMD Serono, Genentech/Roche, GSK, Janssen, Lily, Merck, Novartis, Pfizer, Protagen, Regeneron, Samsung, Sandoz, Sanofi, UCB, S. Dimonaco Employee of: Roche, K. Tuckwell Shareholder of: Roche, Employee of: Roche, M. Klearman Employee of: Genentech, N. Collinson Employee of: Roche, J. H. Stone Grant/research support from: Roche, Genentech, Xencor, Consultant for: Roche, Genentech, Xencor
DOI: 10.1136/annrheumdis-2018-eular.2616