

Background: Connective tissue disease (CTD) often occurs in women of child-bearing age, and flare-ups during pregnancy. Therefore, it is important to manage their disease activities with the treatment which have no influence on fetal growth and development. Among the treatment during pregnancy glucocorticoid is most often used for maintain or control to flare-ups of CTD disease activities. However, prolonged use of glucocorticoid during pregnancy is considered to increase the risk of adverse pregnancy outcomes (APOs) including preterm birth, intrauterine growth restriction, and premature rupture of membrane (PROM) (1, 2).
Objectives: The aim of this study is to reveal the dose of glucocorticoid which influences on APOs.
Methods: We investigated 164 pregnant patients complicated with CTD from March 2006 to January 2019. All these patients were managed their disease activities throughout pregnancy in our institute. APOs including preterm births, light-for-date (LFD) newborns, PROMs in these pregnant patients were examined retrospectively. We analyzed the association between APOs and the incidence or mean dose of glucocorticoid use during pregnancy.
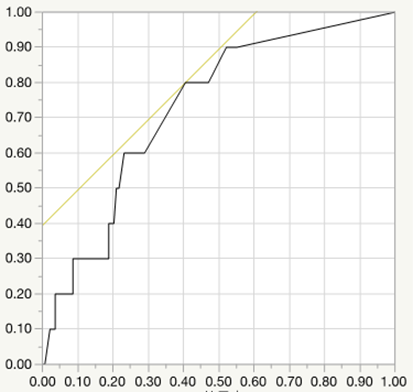
Results: Underlying CTD is Systemic lupus erythematosus (25.3%), Sjogren’s syndrome (24.9%), rheumatoid arthritis (18.1%), Antiphospholipid syndrome (8.8%), mixed connective tissue disease (6.7%), and others. Glucocorticoid was administered in 96 cases, which tended to be earlier gestational week at delivery (37.5±3.0 vs. 38.9±1.5, P<0.01) and lower birth weight of newborns (2601.9±603.0 vs. 3019.0±480.8, P<0.01) significantly. Compared with full-term birth, the cases of preterm birth had higher dose of glucocorticoid during pregnancy significantly (P<0.01). Logistic regression analysis for preterm birth revealed the cut-off value of mean prednisolone dose as 7.5 mg per day (
Conclusion: Glucocorticoid is often used for CTD patients during pregnancy, however, CTD patients who were treated with continuing high dose of glucocorticoid during pregnancy had high risks for APOs such as preterm birth, low birth weight of newborns, preterm PROM. Our data indicated that the cut-off dose of prednisolone values of preterm birth, LFD, preterm PROM was 7.5 mg, 6.7 mg, 5.0 mg per day respectively. It is important for rheumatologists to pay much attention for these risk of glucocorticoid use to CTD patients who hope to conceive and to manage the disease activity with appropriate dose of glucocorticoid. Recently, many immunosuppressants including biologics have been reported to be available for the safety use during pregnancy, and might be helpful to reduce the risk of APOs by glucocorticoid use.
Logistic regression analysis for preterm birth (AUC=0.750, P<0.01, cut-off value=7.5mg/day)
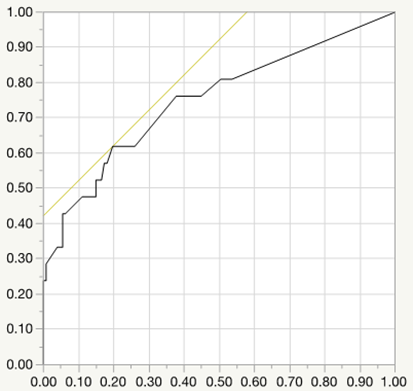
Logistic regression analysis for preterm birth (AUC=0.644, P=0.05, cut-off value=6.7mg/day)
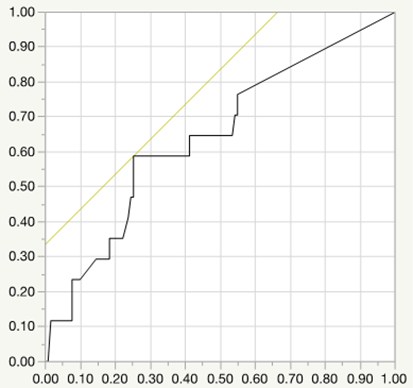
Logistic regression analysis for preterm birth (AUC=0.728, P=0.04, cut-off value=5.0mg/day)
REFERENCES:
[1] Østensen M, Forger F. How safe are anti-rheumatic drugs during pregnancy? Curr Opin Pharmacol 2013;13:470-5.
[2] Gur C, Diav-Citrin O, Shechtman S, et al. (2004) Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol 18:93-101
Disclosure of Interests: None declared
DOI: 10.1136/annrheumdis-2019-eular.4633