

Background: There have been few head-to-head clinical trials comparing different biologic disease-modifying anti-rheumatic drugs (bDMARDs) in patients (pts) with psoriatic arthritis (PsA).
Objectives: To report 24-week (wk) results of a study directly comparing efficacy and safety of ixekizumab (IXE), an IL-17A inhibitor, and adalimumab (ADA), a TNF inhibitor, in bDMARD-naive pts with PsA.
Methods: The study (NCT03151551; SPIRIT-H2H) included pts with active PsA (≥3 TJC + ≥3SJC) and plaque psoriasis (BSA ≥3%) who were bDMARD naive and inadequate responders to csDMARD therapy. Patients were randomised (1:1) to IXE or ADA for 52 wks (on-label dosing based on presence/absence of moderate to severe psoriasis). The primary objective was superiority of IXE vs ADA measured by the proportion of pts achieving both ACR50 and PASI100 responses at wk 24. Key secondary objectives versus ADA at wk 24 were (1) non-inferiority of IXE for ACR50 (noninferiority margin -12%) and (2) superiority of IXE for PASI100. Additional PsA, skin, composite treat-to-target (T2T: MDA, DAPSA 4), PASDAS remission and patient-reported outcomes, and safety were assessed. Nine pts had PASI=0 and BSA≥3% (a medical inconsistency) at baseline; these pts were considered PASI100 responders if PASI=0 and BSA=0 at wk 24. Categorical variables were evaluated using logistic regression analyses with NRI in the ITT population. Continuous variables were analysed using mixed models for repeated measure analysis.
Results: 566 pts were randomised (283 to IXE and 283 to ADA). Baseline demographics and disease characteristics were generally well balanced between groups (Table 1). All primary and key secondary efficacy endpoints at wk 24 were met (Figure). The proportion of pts achieving both ACR50 and PASI100 was significantly greater for IXE than ADA (36% vs 28%; p<0.05). IXE was non-inferior to ADA for ACR50 response and superior for PASI100 response (Figure). While improvements from baseline were achieved with both treatments, significantly better results were seen with IXE vs ADA for skin and composite T2T outcomes, enthesitis resolution (Figure 1), and skin-related quality of life (Table 2). No unexpected safety signals were observed.
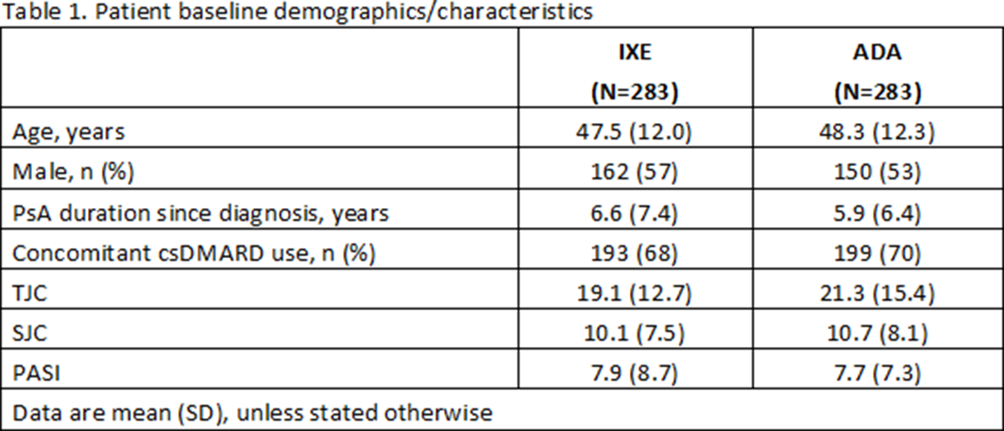
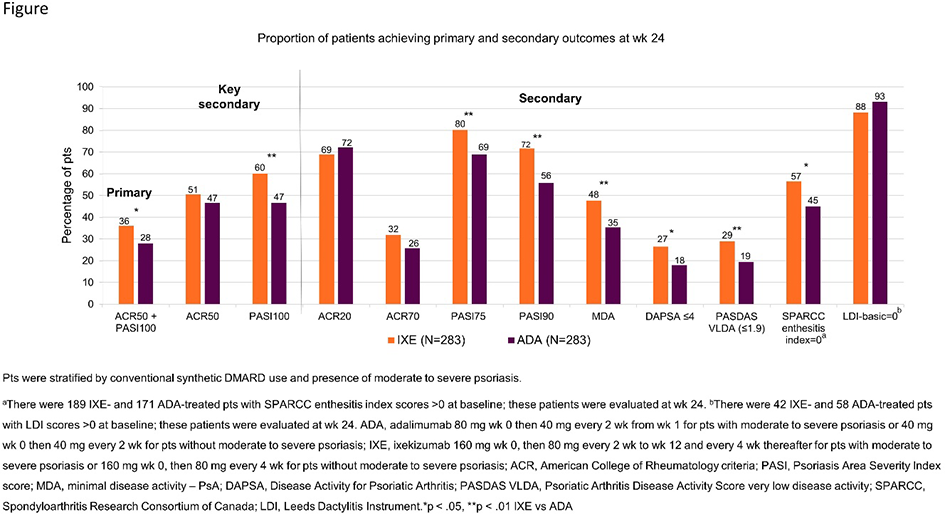
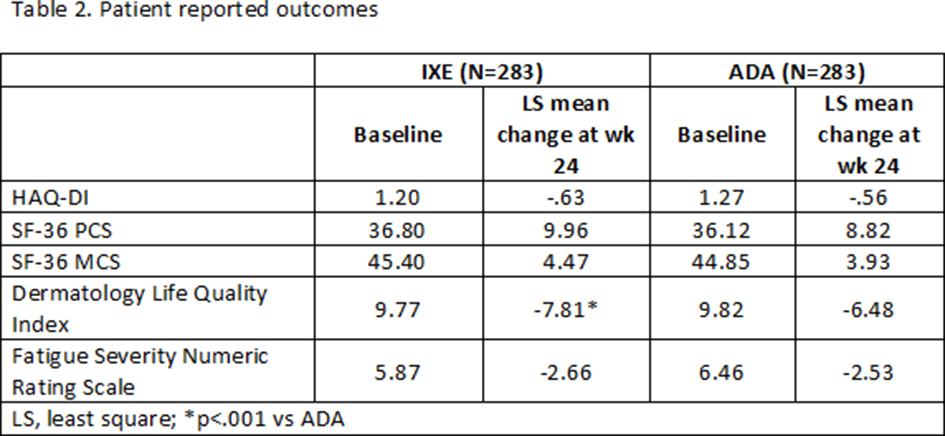
Conclusion: In bDMARD naive pts with active PsA and skin disease, IXE showed superior efficacy to ADA based on simultaneous achievement of ACR50 and PASI100 responses at wk 24. Greater improvements with IXE vs ADA were also attained in individual PsA domains and composite T2T outcomes.
Disclosure of Interests: Philip J Mease Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Leo, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharmaceutical Industries, Inc., and UCB., Consultant for: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Gilead, Janssen, Leo, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharmaceutical Industries, Inc., and UCB., Speakers bureau: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Leo, Merck, Novartis, Pfizer and UCB.., Josef S. Smolen Grant/research support from: AbbVie, Eli Lilly, Janssen, MSD, Pfizer Inc, Roche, Consultant for: AbbVie, Amgen, AstraZeneca, Astro, Celgene, Celtrion, Eli Lilly, GlaxoSmithKline, ILTOO, Janssen, Medimmune, MSD, Novartis-Sandoz, Pfizer Inc, Roche, Samsung, Sanofi, UCB, Speakers bureau: AbbVie, Amgen, AstraZeneca, Astro, Celgene, Celtrion, Eli Lilly, GlaxoSmithKline, ILTOO, Janssen, Medimmune, MSD, Novartis-Sandoz, Pfizer Inc, Roche, Samsung, Sanofi, UCB, Frank Behrens Grant/research support from: AbbVie, Pfizer, Roche, Chugai, Prophylix, Bioline, Novartis, Consultant for: AbbVie, Pfizer, Roche, Chugai, UCB, Bristol-Myers Squibb, Celgene, MSD, Novartis, Biotest, Janssen, Genzyme, Eli Lilly, Speakers bureau: Ad board: AbbVie, Pfizer, Roche, Chugai, UCB, Bristol-Myers Squibb, Celgene, Novartis, Biotest, Janssen, Genzyme, Eli Lilly, Peter Nash Grant/research support from: AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer Inc, Roche, Sanofi, UCB, MSD, Celgene, Gilead, Consultant for: AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer Inc, Roche, Sanofi, UCB, MSD, Celgene, Gilead, Speakers bureau: AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer Inc, Roche, Sanofi, UCB, MSD, Celgene, Gilead, Soyi Liu Leage Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Li Lingnan Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Hasan Tahir Grant/research support from: Novartis, Eli-Lilly, Speakers bureau: AbbVie, Janssen, Eli Lilly, and Novartis, Melinda Gooderham Grant/research support from: Abbvie Inc., Actelion Pharmaceuticals, Akros Pharma Inc., AMGEN Inc., Arcutis Pharmaceuticals Inc., Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Celgene Corporation, Dermira Inc., Eli Lilly and Company, Galderma SA, Glenmark, Janssen Inc., LEO Pharma, MedImmune, Merck and Co., Novartis Pharmaceuticals, Pfizer Inc., Regeneron Pharmaceuticals Inc., Roche Laboratories, Sanofi Genzyme, UCB, Valeant Pharmaceuticals Inc., Consultant for: Akros Pharma Inc., AMGEN Inc., Boehringer Ingelheim International GmbH, Celgene Corporation, Eli Lilly and Company, Janssen Inc., Novartis Pharmaceuticals, Sanofi Genzyme, Valeant Pharmaceuticals Inc., Speakers bureau: Abbvie Inc., Actelion Pharmaceuticals, AMGEN Inc., Boehringer Ingelheim International GmbH, Celgene Corporation, Eli Lilly and Company, Galderma SA, Glenmark, Janssen Inc., LEO Pharma, Novartis Pharmaceuticals, Pfizer Inc., Regeneron Pharmaceuticals Inc., Sanofi Genzyme, Valeant Pharmaceuticals Inc., Eswar Krishnan Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Paul Emery Grant/research support from: Pfizer, MSD, AbbVie, Bristol-Myers Squibb, Roche, Consultant for: Pfizer, MSD, AbbVie, Bristol-Myers Squibb, UCB, Roche, Novartis, Gilead,Samsung, Sandoz and Lilly, Sreekumar Pillai Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Philip Helliwell Grant/research support from: Paid to charity: from AbbVie, Janssen and Novartis, Consultant for: Paid to charity: from AbbVie, Amgen, Pfizer, and UCB and Celgene. Paid to self: from Celgene and Galapagos
DOI: 10.1136/annrheumdis-2019-eular.8709