

Background: Secukinumab (SEC) provided sustained clinical efficacy, and inhibition of radiographic progression over 52 Weeks (Wks) in patients (pts) with psoriatic arthritis (PsA) in the FUTURE 5 study 1 .
Objectives: To report the effect of SEC on radiographic progression at Wk 104 (2 years) in PsA pts in the FUTURE 5 study.
Methods: Adults (N=996) with active PsA, stratified by prior anti-TNF therapy (naïve and inadequate response/intolerance [IR]) were randomised 2:2:2:3 to subcutaneous SEC 300mg with loading dose (LD; 300mg), 150mg LD (150mg), 150mg no LD, or placebo at baseline (BL), Wks 1, 2, 3, 4, and every 4 wks thereafter. Pts could have SEC dose escalated from 150 to 300 mg starting from Wk 52, based on physicians' judgement. Data are shown for pts originally randomised to SEC; 150mg groups include pts who had dose escalated to 300mg. Concomitant MTX (≤25 mg/week) was allowed. Radiographic progression (mean change in van der Heijde-modified total Sharp score [vdH-mTSS] and its components: erosion and joint space narrowing [JSN] scores, was based on hand/wrist/foot X-rays obtained at BL and Wk 104, and assessed by two blinded readers (plus an adjudicator if required). Other efficacy endpoints included ACR20/50, PASI90 and resolution of dactylitis and enthesitis.
Results: Overall, 84.7% (300mg), 82.3% (150mg) and 75.2% (150mg no LD) pts completed 2 years of treatment. A total of 86 (39%) and 92 (41%) pts had their dose escalated to 300mg in the 150mg and 150mg no LD groups, respectively. Inhibition of radiographic progression was sustained with SEC through 2 years (Table 1). Proportions of pts with no radiographic progression (change from BL in mTSS ≤0.5) with SEC were 89.5% (300 mg), 82.3% (150 mg), and 81.1% (150 mg no LD) at 2 years; corresponding proportion of pts for change from BL in mTSS ≤0.0 were: 81.2%, 69.1% and 73.4%, respectively. Clinical responses were also sustained through 2 years (Table 1).
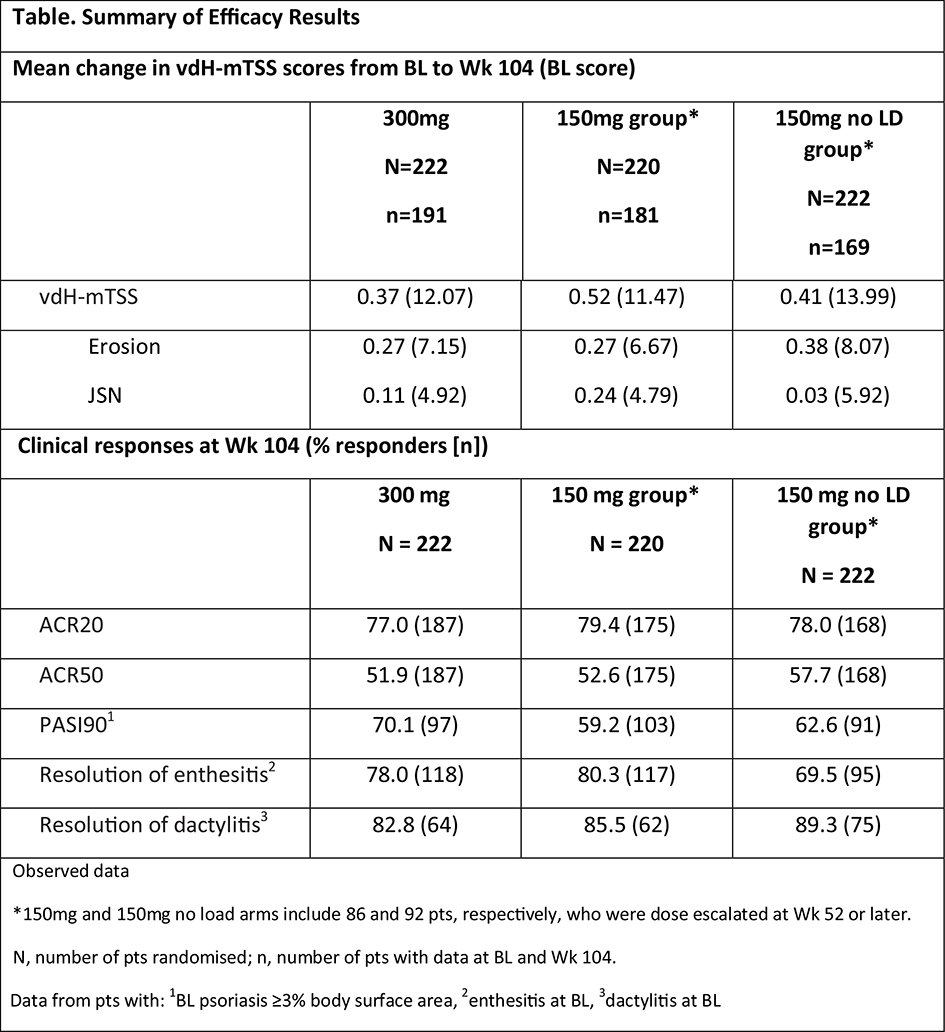
Conclusion: Subcutaneous secukinumab provided sustained inhibition of radiographic progression and sustained clinical responses through 2 years of treatment in pts with active PsA.
REFERENCE:
[1] Mease PJ et al. Arthritis Rheumatol 2018;70(suppl 10).
Disclosure of Interests: Philip J Mease Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Leo, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharmaceutical Industries, Inc., and UCB., Consultant for: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Gilead, Janssen, Leo, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharmaceutical Industries, Inc., and UCB., Speakers bureau: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Leo, Merck, Novartis, Pfizer and UCB.., Robert B.M. Landewé: None declared, Proton Rahman Grant/research support from: Investigator for Janssen Research & Development, LLC and Novartis, Consultant for: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly,Novartis, and UCB, Speakers bureau: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, and Novartis, Hasan Tahir Grant/research support from: Novartis, Eli-Lilly, Speakers bureau: AbbVie, Janssen, Eli Lilly, and Novartis, Atul Singhal Grant/research support from: AAbbVie, Gilead, Sanofi, Regeneron, Amgen, Roche, BMS, Janssen, Lilly, Novartis, Pfizer, UCB, Astra Zeneca, MedImmune, FujiFilm, Nichi-Iko, Mallinckrodt, Speakers bureau: AbbVie, Elke Boettcher Consultant for: Amgen, Roche, Eli Lilly, Pfizer, MSD, Novartis, Speakers bureau: Amgen, Roche, Eli Lilly, Pfizer, MSD, Novartis, Sandra Navarra Speakers bureau: Astellas, Johnson & Johnson, Novartis, Pfizer, Aimee Readie Shareholder of: Novartis, Employee of: Novartis, Shephard Mpofu Shareholder of: Novartis, Employee of: Novartis, Eumorphia Maria Delicha Consultant for: Novartis, Luminita Pricop Shareholder of: Novartis, Employee of: Novartis, Désirée van der Heijde Consultant for: AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi, Eli-Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, Union Chimique Belge
DOI: 10.1136/annrheumdis-2019-eular.8808