

Background: In the SENSCIS trial, nintedanib reduced the progression of interstitial lung disease associated with systemic sclerosis (SSc-ILD) compared with placebo, as demonstrated by a significantly lower rate of decline in forced vital capacity (FVC) over 52 weeks (primary endpoint).
Objectives: To assess the effect of nintedanib on the rate of decline in FVC in the SENSCIS trial across pre-specified subgroups defined by baseline characteristics.
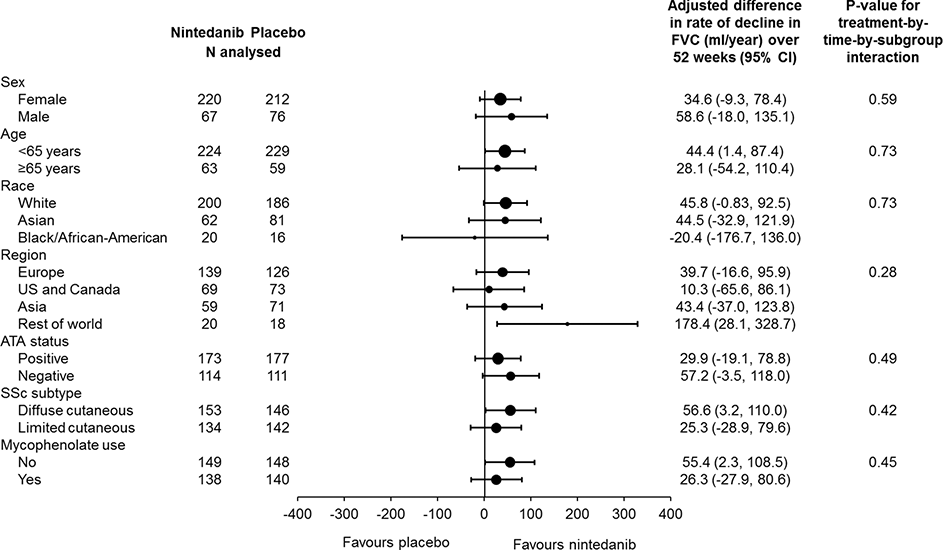
Methods: Patients with SSc-ILD with onset of first non-Raynaud symptom <7 years before screening and ≥10% fibrosis of the lungs on a high-resolution computed tomography scan were randomised to receive nintedanib 150 mg bid or placebo double-blind. The annual rate of decline in FVC (ml/year) assessed over 52 weeks (primary endpoint) was analysed in the overall population using a random coefficient regression model (with random slopes and intercepts) including anti-topoisomerase I antibody (ATA) status, age, height, gender and baseline FVC as covariates. Analyses in subgroups by baseline characteristics included additional terms for treatment-by-subgroup and treatment-by-subgroup-by-time interaction.
Results: A total of 576 patients were treated (288 in each group). Most (75.2%) of patients were female, 51.9% had diffuse cutaneous SSc, and 48.4% were taking mycophenolate at baseline. Mean ± SD age was 54.0 ± 12.2 years and 21.4% of patients were aged ≥65 years. Nintedanib had a consistent effect on reducing the rate of FVC decline across pre-specified subgroups defined by baseline characteristics (p>0.05 for all treatment-by-time-by-subgroup interactions) (figure).
Conclusion: Nintedanib is effective at reducing ILD progression in a broad range of patients with SSc-ILD.
Disclosure of Interests: Oliver Distler Grant/research support from: Prof. Distler received research funding from Actelion, Bayer, Boehringer Ingelheim and Mitsubishi Tanabe to investigate potential treatments of scleroderma and its complications, Consultant for: Prof. Distler has/had consultancy relationship within the last 3 years with Actelion, AnaMar, Bayer, Boehringer Ingelheim, ChemomAb, espeRare foundation, Genentech/Roche, GSK, Inventiva, Italfarmaco, iQvia, Lilly, medac, MedImmune, Mitsubishi Tanabe Pharma, Pharmacyclics, Novartis, Pfizer, Sanofi, Serodapharm and UCB in the area of potential treatments of scleroderma and its complications. In addition, he had/has consultancy relationship within the last 3 years with A. Menarini, Amgen, Abbvie, GSK, Mepha, MSD, Pfizer and UCB in the field of arthritides and related disorders, Kristin Highland Grant/research support from: Kristin Highland is a site PI for the SENSCIS trial (Dr Highland’s institution has the contract for the study) which is funded by Boehringer Ingelheim., Consultant for: Kristin Highland is a paid consultant for Boehringer Ingelheim through her role sitting on the steering committee., Speakers bureau: Kristin Highland is on the speakers’ bureau for Boehringer Ingelheim., Martina Gahlemann Employee of: Employee of Boehringer Ingelheim
, Arata Azuma Consultant for: Arata Azuma has received personal fees from Boehringer Ingelheim, Shionogi & Co., Ltd, Taiho Pharmaceutical Co., Ltd, and Asahikasei Pharma Co., Aryeh Fischer Grant/research support from: Aryeh Fischer has received a grant from Boehringer Ingelheim (Consultant/steering committee member/principal investigator on clinical trials)., Consultant for: Aryeh Fischer has received personal fees from Boehringer Ingelheim (Consultant/steering committee member/principal investigator on clinical trials), Genentech-Roche (Consultant/steering committee member/principal investigator on clinical trials), Pfizer (Consultant) and Genentech (Consultant)., Maureen Mayes Grant/research support from: Maureen Mayes is a clinical trial investigator for Boehringer-Ingelheim; Galapagos, Reata, Sanofi, Merck-Serono, Consultant for: Maureen Mayes is a member of scientific advisory boards for Galapagos NV (Pharma), Boehringer-Ingelheim, Mitsubishi-Tanabe, Astellas: Grant Review Board for Actelion., Speakers bureau: Maureen Mayes received personal fees for being a conference speaker on the use of autoantibodies in connective tissue diseases for Medtelligence, Ganesh Raghu Grant/research support from: Ganesh Raghu is the principal investigator for IPF net studies and is a steering committee member for IPF net studies for the NIH., Consultant for: Ganesh Raghu is a consultant for Boehringer Ingelheim, Bellerophan, Biogen, BMS, Fibrogen, Gilead, Nitto, Revistan, Promedior, Sanofi, Veracyte and Roche-Genentech; and a consultant and chair of the DSMB for Avalyn., Wiebke Sauter Employee of: Wiebke Sauter is an employee of Boehringer Ingelheim, Mannaig Girard Employee of: Mannaig Girard is an employee of Boehringer Ingelheim, Margarida Alves Employee of: Employee of Boehringer Ingelheim, Emmanuelle Clerisme-Beaty Employee of: Emmanuelle Clerisme-Beaty is an employee of Boehringer Ingelheim, Susanne Stowasser Employee of: Susanne Stowasser is an employee of Boehringer Ingelheim, Masataka Kuwana Grant/research support from: Actelion, Consultant for: Chugai, Reata, GlaxoSmithKline, Bayer, Boehringer-Ingelheim, Corpus, CSL-Berling, Mochida, Speakers bureau: Actelion, Pfizer, Bayer, Nippon Shinyaku, Chugai, Toby M Maher Shareholder of: Has stock options or bond holdings in a for-profit corporation in Apellis, Grant/research support from: Received funds from BI advisory board participation and conference travel. Received research funding and/or consulting fees or other remuneration from GSK, UCB, AstraZeneca, Roche, Bayer, Biogen Idec, Cipla, Prometic, and Sanumed, Consultant for: Toby Maher has received consultancy or speakers fees from Apellis, AstraZeneca, Bayer, Biogen Idec, Boehringer Ingelheim, Galapagos, GlaxoSmithKline R&D, Indalo, Pliant, ProMetic, Roche, Samumed, and UCB; and has received consultancy fees from Galecto
DOI: 10.1136/annrheumdis-2019-eular.3323