

Background: Autologous hematopoietic stem cell transplantation (HSCT) has shown superior efficacy to cyclophosphamide pulse therapy in systemic sclerosis (SSc) but its application is hampered by high treatment related mortality (TRM). To date factors, other than smoking, predicting TRM are unknown.
Objectives: To describe event-free survival and TRM after HSCT and to evaluate the predictive value of baseline characteristics. Event-free survival was defined as the absence of mortality or major organ failure (i.e. cardiac failure (left ventricular ejection fraction (LVEF) < 30%, respiratory failure (resting PaO2 < 8kPa/60 mm Hg or PaCO2 > 6,7 kPa or chronic oxygen supplementation), or renal failure requiring dialysis.
Methods: All patients who started with the procedure of HSCT for SSc in the Netherlands between 1.1.1998 and 31.12.2016 were included. Except in 4 patients, eligibility criteria and procedures of the ASTIS trial were used (1). A Kaplan-Meier event-free survival curve was constructed. All deceased patients were discussed in a consensus meeting, to determine the cause of death as TRM, progression of SSc or other. The univariate association of TRM and event-free survival with baseline characteristics (gender, age, disease duration, smoking ever, FVC, DLCO, LVEF) was examined by Cox regression analysis.
Results: 92 patients were included, including 4 patients with limited cutaneous subtype who were treated because of interstitial lung disease. The median (IQR) follow-up time was 4.5 (2.3 – 12.3) years. Twenty patients died during the observation period of whom 10 (10.8%, 9 males) in the first year because of TRM. Twelve patients (13.1%) developed major organ failure (6 lung, 3 kidney and 3 heart) of whom 8 died during the observation period. Event-free survival after five and ten years was 68% (40/59 patients) and 59% (30/51), respectively. TRM was significantly associated with male gender (HR (95% CI): 8.7 (1.1 -68.9), but not with smoking; events were associated with male gender, older age and LVEF <50% at baseline (HR: 4.08 (1.5 – 11.0); 1.1 (1.0-1.1) and 4.7 (1.1-20.6), respectively).
demographic and disease related factors
| Male/female | 49/43 |
| LcSSc/DcSSc | 4/88 |
| Age at HSCT mean (SD) (year) | 46.8 (10.3) |
| Disease duration at HSCT mean (SD)(year) | 2.4 (2.4) median 1.6 |
| mRSS mean (SD) | 26 (10.1) |
| Antibodies: ANA, ATA, Anti-RNAP positive | 88/92; 34/92; 1/92 |
| Ever smoker | 54/84 |
| FVC as% pred. mean (SD) | 84.9 (22.7) |
| DLCO as% pred mean (SD) | 55.1 (16.2) |
| LVEF < 50% (by echo) | 3/92 |
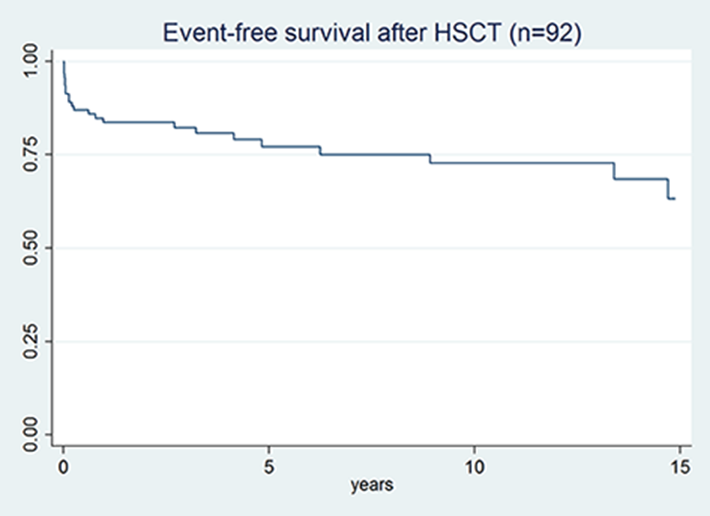
Conclusion: In this national cohort study of HCST in patients with SSc and poor prognosis we observed a median event-free survival of 4.3 years. TRM was 10,8% and major events occurred in 13%. TRM was correlated to male gender. Low LVEF, male gender and older age were correlated with major events.
REFERENCES:
[1] van Laar JM, Farge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311(24):2490-8.
Disclosure of Interests: Sandra van Bijnen: None declared, Maaike Boonstra: None declared, Cornelia van den Enden: None declared, Julia Spierings Grant/research support from: Boehringer Ingelheim, Anne Schouffoer: None declared, Jacob M. van Laar Grant/research support from: Genentech, Consultant for: F. Hoffmann-La Roche, Thomas Huizinga Consultant for: Merck, UCB, Bristol Myers Squibb, Biotest AG, Pfizer, GSK, Novartis, Roche, Sanofi-Aventis, Abbott, Crescendo Bioscience Inc., Nycomed, Boeringher, Takeda, Zydus, Epirus, Eli Lilly, Alexandre Voskuyl: None declared, Walter van der Velden: None declared, Frank van den Hoogen: None declared, Jeska de Vries: None declared, Madelon Vonk Grant/research support from: Madelon Vonk has received unrestricted research funds from Actelion and Therabel, Consultant for: Madelon Vonk was a consultant for Actelion, Boehringer-Ingelheim, Speakers bureau: Actelion, Boehringer-Ingelheim, Roche
DOI: 10.1136/annrheumdis-2019-eular.5380