

Background: With a ‘treat to target’ approach in RA, guidelines recommend tailored monitoring of disease activity using validated composite instruments, such as the disease activity score (DAS) 28. While response assessment at 24 weeks is the standard in clinical trials, assessment as early as 12 weeks is recommended. There is limited evidence assessing the relative efficacy of TIMs following a ‘treat to target’ strategy.
Objectives: To evaluate the relative efficacy of intravenous (IV) and subcutaneous (SC) tocilizumab plus a conventional disease modifying antirheumatic drug (cDMARD) to other TIMs plus a cDMARD in TIM-naïve or mixed (<20% TIM-experienced) adults with moderate to severe RA. Efficacy was defined as achieving remission according to a DAS28 score <2.6 at 12 and 24 weeks.
Methods: Randomized controlled trials (RCTs) were selected from a recent systematic literature review conducted by the Institute for Clinical and Economic Review (ICER), as well as from trials for upadacitinib (SELECT-NEXT, SELECT-COMPARE), which were not included in the ICER 2017 report. RCTs that compared TIMs to each other or placebo were included. Treatments included Janus kinase (JAK) inhibitors (upadacitinib, baricitinib, and tofacitinib), tumor necrosis factor alpha inhibitors (TNFi; adalimumab, certolizumab pegol, golimumab, and infliximab), and other non-TNFis (rituximab, sarilumab, tocilizumab, and abatacept). A Bayesian NMA was performed in OpenBUGS and R using a fixed effects model. Model selection was based on deviance information criterion. Forest plots of odds ratios (OR) are presented.
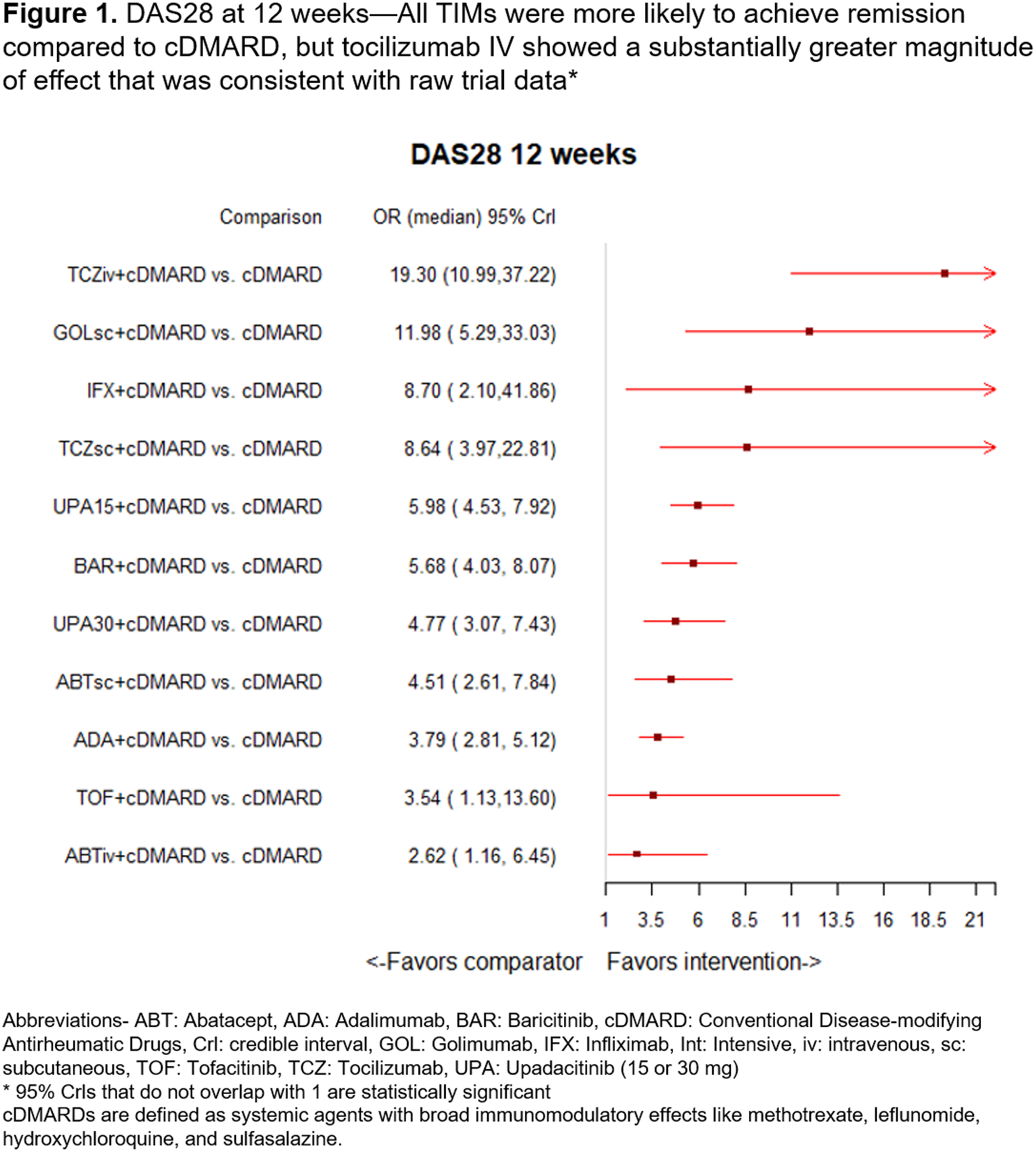
Results: In the 12-week analysis, 15 trials were included with a pooled study population of 9,154 patients. Populations were similar across trials and predominantly female (mean 78%, range 39-87%), with a baseline mean age of 52 years (range 47-56), mean disease duration of 8 years (range 2-11), and mean DAS28 score of 6 (range 5-7). In the 12-week analysis, compared to cDMARD, all TIMs were more likely to achieve remission (statistically significant), but tocilizumab IV showed a substantially greater magnitude of effect (OR=19.3, 95% Crl=10.99, 37.22) which was consistent with raw trial results (
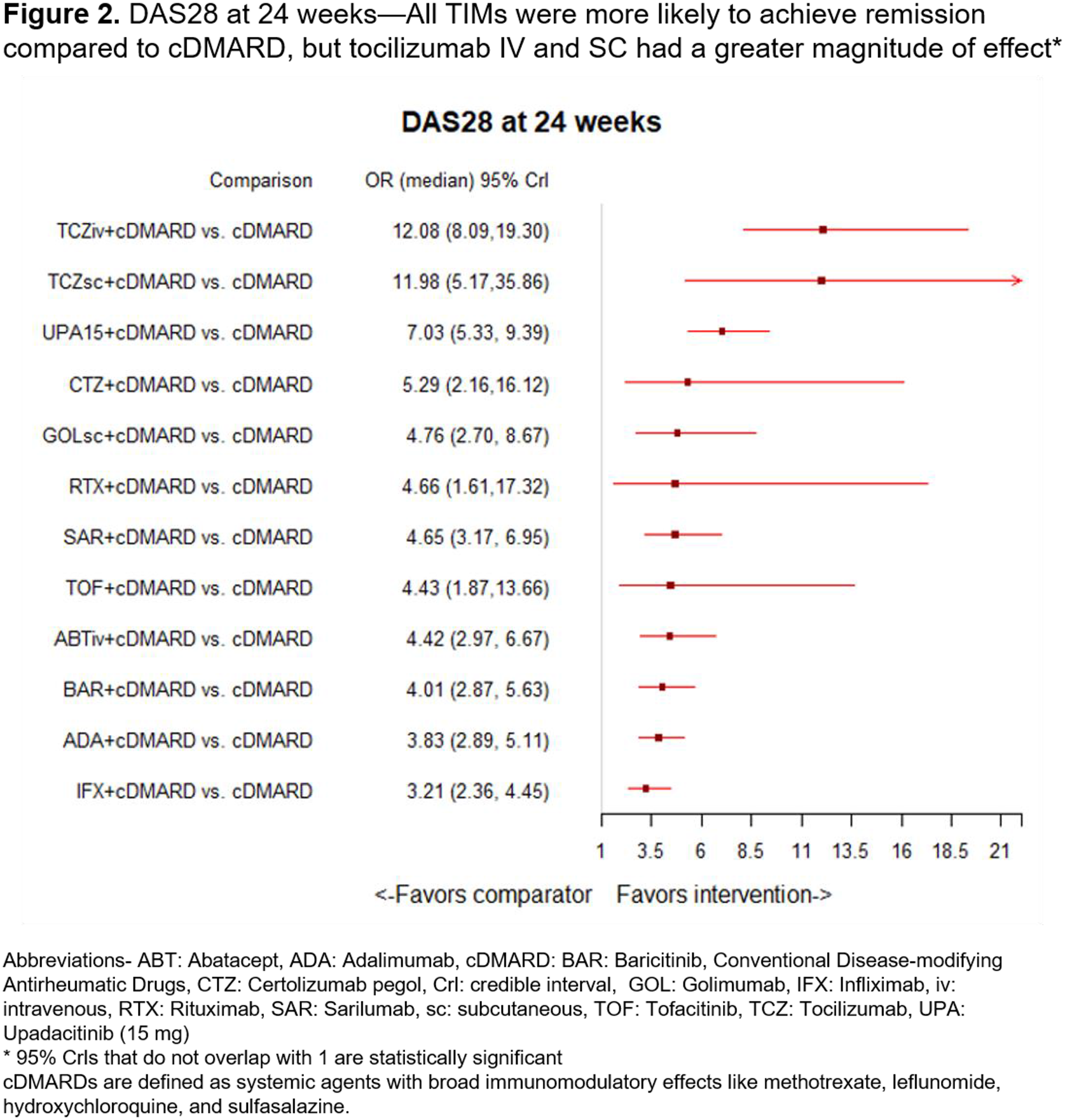
In the 24-week analysis, 21 trials were included in the analysis with a pooled study population of 12,180 patients. Patient characteristics were the same as the 12 week analysis. Compared to cDMARD, all TIMs were more likely to achieve remission (statistically significant), with tocilizumab IV and SC showing a greater magnitude of effect (OR=12.08, Crl=8.09-18.30 and OR=11.98, Crl=5.17-35.86, respectively) (
Conclusion: Results of this NMA demonstrate that tocilizumab is associated with a greater likelihood of remission (DAS28 <2.6) at 12 and 24 weeks compared to most other TIMs including new JAK inhibitors, when used in combination with a cDMARD among TIM-naïve/mixed patient populations.
Acknowledgments: This study was sponsored and funded by Genentech, Inc.
Disclosure of Interests: Jennie H. Best Shareholder of: Genentech, Inc., Employee of: Genentech, Inc., Jennifer Uyei: None declared, Joseph Dang Shareholder of: Genentech, Inc., Employee of: Genentech, Inc., Yilin Jiang: None declared, Rajpal Singh: None declared, Pinar Bilir: None declared, Andreas Karabis: None declared, Julie Munakata: None declared, William Reiss Shareholder of: Genentech, Inc., Employee of: Genentech, Inc.