

Background: PF-06410293 (ADL-PF) is an adalimumab biosimilar approved for the treatment of several inflammatory and autoimmune indications. 1 The efficacy, safety and immunogenicity of ADL-PF and reference adalimumab sourced from the European Union (ADL-EU) in patients with rheumatoid arthritis (RA) have been demonstrated to be similar in a randomised controlled trial up to 26 weeks (wks; treatment period 1 [TP1]). 2
Objectives: To evaluate the efficacy, safety and immunogenicity of ADL-PF and ADL-EU in patients with moderate to severe RA on longer-term treatment, and following a treatment switch from ADL-EU to ADL-PF in a subset of patients.
Methods: This multinational, randomised, double-blind, parallel-group study compared ADL-PF and ADL-EU in essentially biologic-naïve patients with active RA despite methotrexate (MTX) (NCT02480153). In TP1, patients were randomised (1:1) to ADL-PF or ADL-EU (40 mg subcutaneous injection every 2 wks) for 26 wks while continuing MTX (10–25 mg/wk). The primary endpoint was achievement of American College of Rheumatology response (ACR20) at Wk 12. At Wk 26, the start of treatment period 2 (TP2), patients receiving ADL-EU were blindly re-randomised (1:1) to remain on ADL-EU or switch to ADL-PF for 26 wks while patients receiving ADL-PF continued treatment in a blinded manner. Secondary efficacy endpoints at Wks 26, 30, 36, 44 and 52 (ACR20/50/70, European League Against Rheumatism [EULAR] response, Disease Activity Score [DAS] 28-4[CRP] <2.6 and ACR/EULAR defined remission), safety events and percentage of patients with anti-drug antibodies (ADA) were assessed.
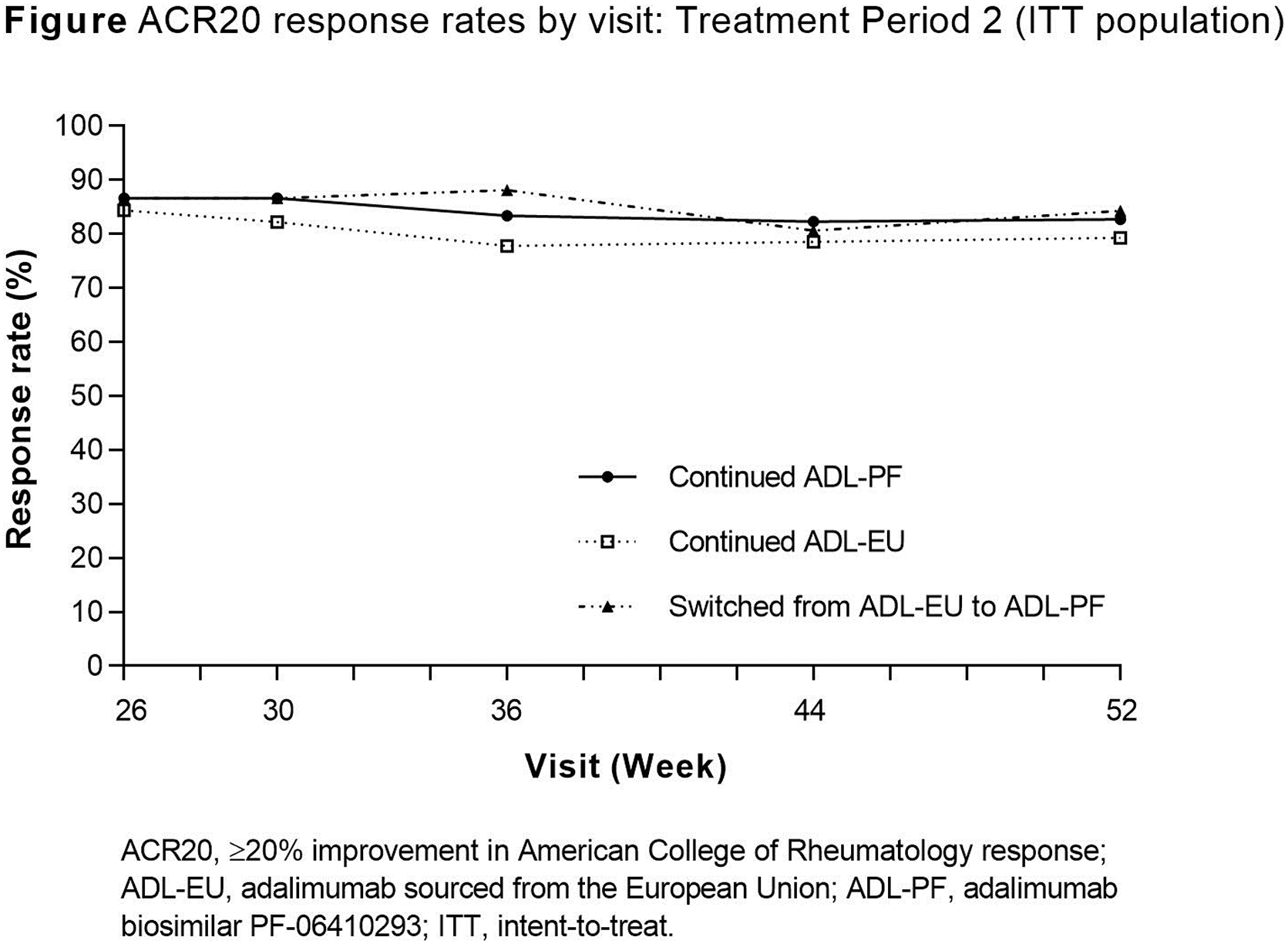
Results: In TP1, 597 patients were randomised to ADL-PF (n=297) or ADL-EU (n=300). At Wk 26, 552 patients were re-randomised for TP2 (continued ADL-PF, n=283; continued ADL-EU, n=135; switched from ADL-EU to ADL-PF, n=134). Patients who demonstrated at least minimal efficacy continued in TP2. Observed ACR20 rates were comparable between treatment groups at all visits during TP2 ( Figure ). Other measures of deep response (ACR70, EULAR good response, DAS28-4(CRP) <2.6 and ACR/EULAR defined remission) showed maintained efficacy during TP2 in all treatment groups. Incidences of treatment-emergent adverse events were comparable between treatment groups ( Table ). Overall, incidences of ADA through Wk 52 were comparable between treatment groups (47.3%, 54.1% and 45.9% for patients who continued ADL-PF, continued ADL-EU or switched from ADL-EU to ADL-PF, respectively). In patients who switched from ADL-EU to ADL-PF compared with patients who continued ADL-EU, the increase in ADA incidence over TP2 was 0.8% (from 45.1% to 45.9%) versus 6.7% (from 47.4% to 54.1%), respectively.
Conclusion: TP2 results demonstrated comparable efficacy, safety and immunogenicity between ADL-PF and ADL-EU was maintained up to Wk 56 and was unaffected by a blinded switch from ADL-EU to ADL-PF at Wk 26.
REFERENCES:
[1]Pfizer Inc, 2019.
[2]Fleischmann RM et al, Arthritis Res Ther 2018;20:178.
All-causality TEAEs: Treatment Period 2 (Safety population)
| Continued ADL-PF
| Continued ADL-EU
| Switched from ADL-EU to ADL-PF
|
|
|---|---|---|---|
| Number of AEs | 243 | 112 | 100 |
| Patients with events, n (%) | |||
| AEs | 123 (43.5) | 60 (44.4) | 51 (38.3) |
| Serious AEs | 4 (1.4) | 6 (4.4) | 3 (2.3) |
| ≥ Grade 3 AEs | 7 (2.5) | 7 (5.2) | 4 (3.0) |
| TEAEs leading to treatment discontinuation | 6 (2.1) | 8 (5.9) | 2 (1.5) |
| Deaths | 0 | 0 | 0 |
ADL-EU, adalimumab sourced from the European Union; ADL-PF, adalimumab biosimilar PF-06410293; AE, adverse event; TEAE, treatment-emergent AE.
Acknowledgments: Medical writing support, provided by Jacqui Oliver of Engage Scientific Solutions. The study was funded by Pfizer.
Disclosure of Interests: Roy Fleischmann Grant/research support from: AbbVie, Akros, Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer, IngelhCentrexion, Eli Lilly, EMD Serono, Genentech, Gilead, Janssen, Merck, Nektar, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Roche, Samsung, Sandoz, Sanofi Genzyme, Selecta, Taiho, UCB, Consultant of: AbbVie, ACEA, Amgen, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, Novartis, Pfizer, Sanofi Genzyme, UCB, Daniel Alvarez Shareholder of: Pfizer, Employee of: Pfizer, Amy Bock Shareholder of: Pfizer, Employee of: Pfizer, Carol Cronenberger Shareholder of: Pfizer, Employee of: Pfizer, Ivana Vranic Shareholder of: Pfizer, Employee of: Pfizer, Wuyan Zhang Shareholder of: Pfizer, Employee of: Pfizer, Rieke Alten Grant/research support from: Pfizer, Galapagos, Galapagos NV, Gilead, Gilead Sciences, Inc., Novartis, Consultant of: Pfizer, Speakers bureau: Pfizer