

Background: Little is known about effectiveness of certolizumab (CTZ) in clinical practice, especially in patients with inadequate response to prior biologics.
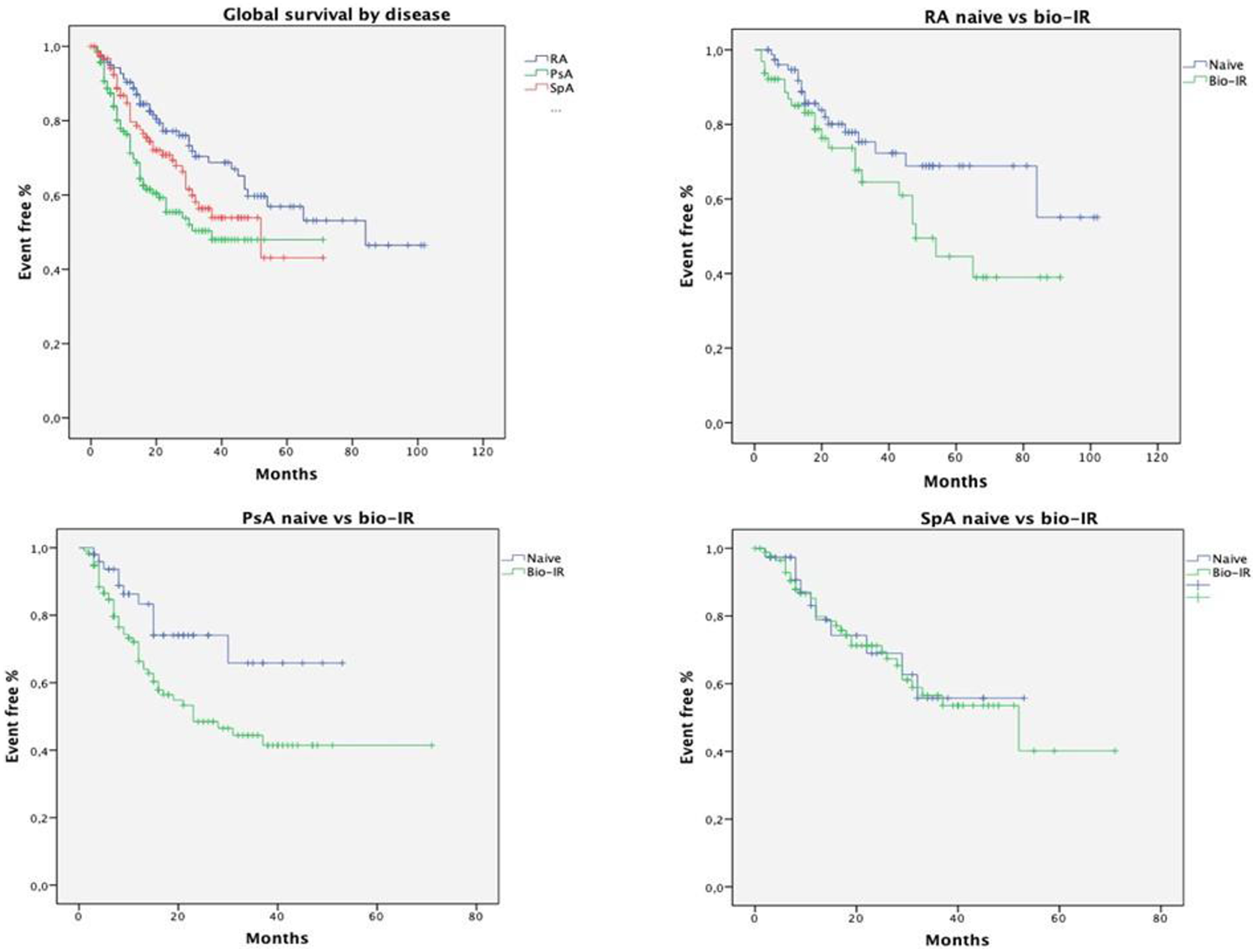
Objectives: To estimate the survival rate of CTZ in RA, PsA or SpA cohorts from the registry BIOPURE. Secondary endpoint was the changes of clinical outcomes from baseline at 6 and 12 months for each disease.
Methods: We analyzed longitudinal data of consecutive patients, affected with RA, PsA or SpA starting a treatment with CTZ recorded into the web-based Apulian registry BIOPURE. Demographic and disease related characteristics were collected at baseline, 6 and 12 months. Drug survival was evaluated by Kaplan-Meier life table analysis. Estimates hazard ratios (HRs, 95% confidence intervals (CI)) of drug discontinuation adjusted for patient’s demographics, disease characteristics and prior biologic treatments were computed by Cox-regression models. Differences of DAS28, DAPSA and BASDAI among baseline, 6 and 12 months were estimated by T-test.
Results: 506 patients were included in this analysis (
Conclusion: In real-life settings CTZ has shown a good effectiveness also in Bio-IR patients. Unlike other TNF-inhibitors, the clinical response and the survival rate were also meaningful in RA patients.
| RA
| PsA
| SpA
|
|
|---|---|---|---|
| Age (mean ± SD) | 54.5 ±12 | 50.6 ±12 | 52.0 ±11 |
| Female | 82.9 % | 74.6 % | 56.3 % |
| BMI (mean) | 25.9 ± 5 | 28.4 ± 5 | 26.7 ± 5 |
| Dis Durat months (mean ± SD) | 46 ± 14 | 106 ± 82 | 97 ± 92 |
| Naive | 53.9 % | 32.8 % | 28.5 % |
| Prior biologics | 52.9 % | 75.0 % | 71.1 % |
| Glucocorticoids | 55.9 % | 39.7 % | 39.4 % |
| DMARDs | 72.4 % | 52.4 % | 43.8 % |
| DAS28 (mean ± SD) | 4.8 ± 1.5 | 3.6 ± 1.2 | 3.7 ± 1.3 |
| BASDAI (mean ± SD) | 5.2 ± 2 | ||
| DAPSA (mean ± SD) | 19.7 ±10 | ||
| HAQ (mean ± SD) | 1.2 ± 0.7 | 1.1 ± 0.6 | 1.2 ± 0.7 |
| RF/ACPA + | 72.4 % |
Disclosure of Interests: Florenzo Iannone Consultant of: Speaker and consulting fees from AbbVie, Eli Lilly, Novartis, Pfizer, Roche, Sanofi, UCB, MSD, Speakers bureau: Speaker and consulting fees from AbbVie, Eli Lilly, Novartis, Pfizer, Roche, Sanofi, UCB, MSD, Nicola Maruotti Speakers bureau: Pfizer, Angelo Semeraro Speakers bureau: Sanofi, Roche, AbbVie, BMS, MSD, Novartis, Romano Bucci Speakers bureau: Pfizer, Sanofi, MSD, BMS, Giorgio Carlino Speakers bureau: Pfizer, Janssen, AbbVie, MSD, BMS., Leonardo Santo Consultant of: AbbVie, MSD, Novartis UCB outside this work, Speakers bureau: AbbVie, MSD, Novartis UCB outside this work, Laura Quarta: None declared, Carmelo Zuccaro Consultant of: MSD, AbbVie, Novartis, Pfizer, Janssen outside this work, Speakers bureau: MSD, AbbVie, Novartis, Pfizer, Janssen outside this work, Giuseppina Santacesaria: None declared, Marco Fornaro: None declared, Francesco Paolo Cantatore: None declared