

Background: EGPA is a small vessel vasculitis characterised by the presence of tissue eosinophilia, necrotising vasculitis and granulomatous inflammation 1 . Typically, a prodromal asthmatic phase, leads to an eosinophilic stage, which can evolve to include the presence of vasculitis with renal manifestations. In the recent randomised, placebo-controlled MIRRA trial for relapsing and refractory EGPA, adjuvant therapy with anti-IL5 mAB Mepolizumab [MEPO] at 300mg s/c monthly, accrued longer times in remission, reduced steroid exposure and reduced relapse rates 2 .
Objectives: The aim of our study was to analyse the response and outcome for EGPA patients who received 100mg s/c of MEPO monthly for a minimum of 52 weeks, with particular focus on the steroid minimisation benefits.
Methods: This retrospective, descriptive study analysed 13 patients with EGPA, who received 100mg s/m monthly MEPO therapy under the eosinophilic asthma care-pathway. Time points of assessment included MEPO commencement [M0] and 12 [M12] months.
EGPA patients receiving Mepolizumab therapy for one year [100mg s/c]
| Demographics All [n=13] | |
| Gender ratio M/F | 4M:9F |
| ANCA positive/ negative | ANCA: 3MPO, 1 PR3 positive/ 9 ANCA negative |
| Age of diagnosis of asthma | 35 yrs [IQR 28.5-40] |
| Age of diagnosis of EGPA | 47 yrs [IQR 43.5-53.5] |
| Median age | 51 yrs [IQR 47.5- 60.5] |
| EGPA disease characteristics N=13 [%] | |
| Asthma 13 [100] | |
| Serum eosinophilia or biopsy evidence [N= 12] 12 [100] | |
| Pulmonary infiltrates, non-fixed 8[61.5] | |
| Neuropathy, mono/poly 4[30.7] | |
| Sino-nasal abnormality 12[92.3] | |
| Glomerulonephritis 3[23] | |
| Cardiovascular 4[30.7] | |
| Prior Immunosuppressants N=13 [%] | |
| Steroids | 13[100%] |
| Cyclophosphamide 6[46%] | |
| Rituximab | 6[46%] |
| Azathioprine | 10[77%] |
| Mycophenolate mofetil | 8[62%] |
| Methotrexate | 4[31%] |
| Campath 1[7%] | |
| Response to therapy M0 [%] Post M12 [%] | |
| Prednisolone dose N= 13 | |
| Mean ±SD 18.925 mg ±11.44 10.575mg ± 5.85 | |
| Eosinophil count X10 9 /L N=13 | |
| Mean ±SD 0.415mg ±0.25 0.035±0.039 | |
| Asthma Control Questionnaire [ACQ] N=5 | |
| Mean ±SD 2.92 ±1.27 1.31± 0.79 | |
| BVAS N= 13 | |
| Mean ±SD 7.307±6.29 2.2307±1.69 | |
| Creatinine N=9 | |
| Mean ±SD 68.44±15.03 69.11±17.84 | |
| Continuation of anti-IL5 therapy N=13 12/13 [92.3%] |
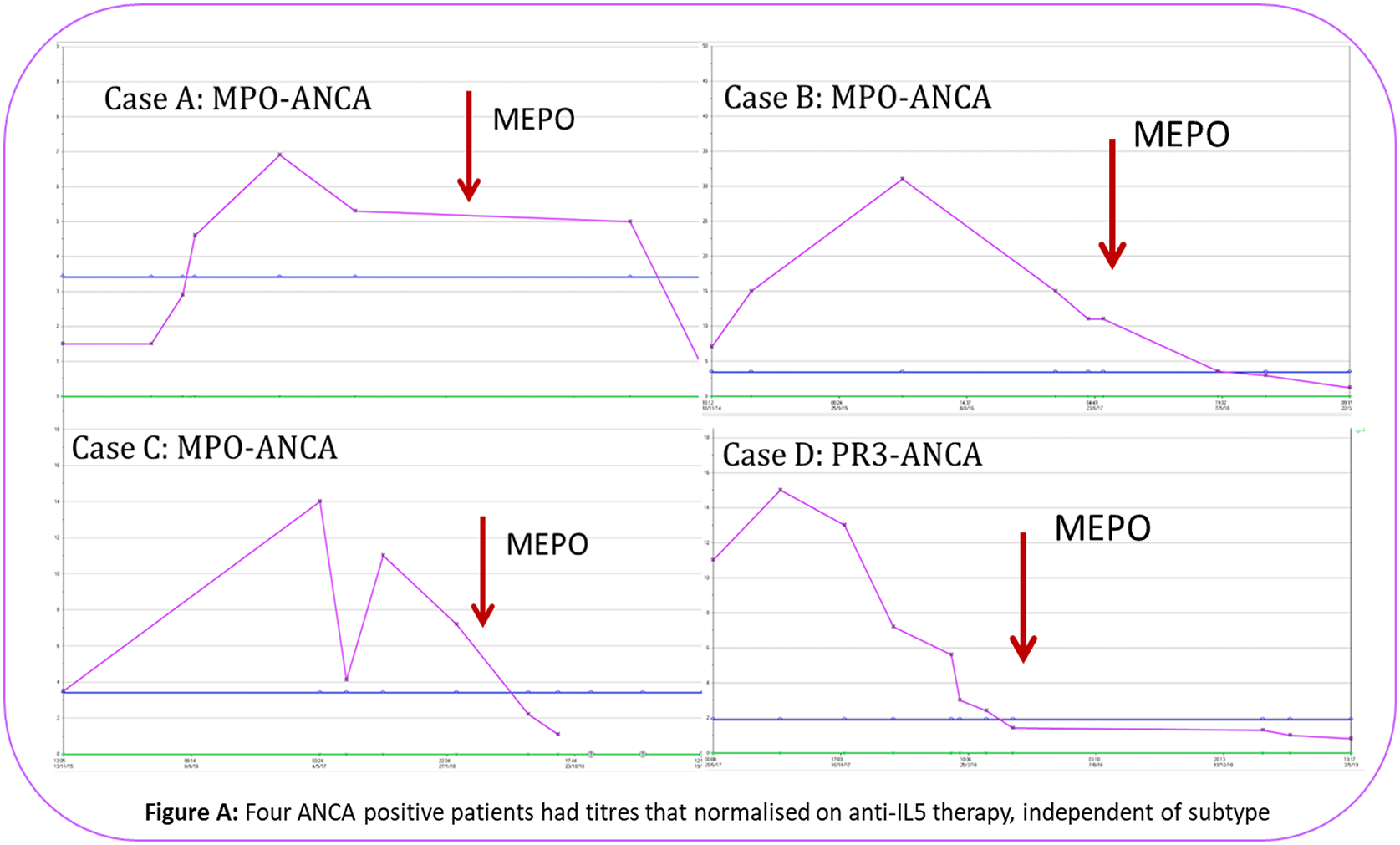
Conclusion: The relapsing nature of EGPA places a potential dependency of therapy on steroids for asthmatic and vasculitic flares. This underscores the importance of targeted pathway specific biologic therapy to minimise steroid exposure, prevent tissue damage and ensure early response to therapy. This study demonstrates that anti-IL5 serves as a favourable model with steroid minimisation, improvement in asthma control questionnaire, reduction in BVAS and eosinophil counts at the 100mg s/c dosage. ANCA positive serology normalised in all four patients, independent of subtype. Well tolerated, it demonstrated considerable clinical benefit, with 12 patients [92.3%] continuing anti-IL5 therapy beyond 12 months.
| Long term plan > 12 months N=13 [%] Current Months Adjuvant therapy 12M |
| 1 Continue
|
| 2 Switched Benralizumab 26 MMF [+], IVIG [-] |
| 3 Continue 18 |
| 4 Switched Benralizumab 14 |
| 5 Discontinued Rituximab 12 MTX |
| 6 Continue 14 |
| 7 Continue 24 MMF Reduced |
| 8 Continue 18 MTX [+] |
| 9 Continue 15 MMF [-] |
| 10 Continue 14 |
| 11 Continue 13 |
| 12 Continue 13 Aza |
| 13 Continue 12 |
REFERENCES:
[1]J.C.Jenette, et al Revised International Chapel Hil Consensus Conference Nomenclature of Vasculitides. 65 , 1–11 (2013).
[2]Wechsler, M. E. et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 376 , 1921–1932 (2017).
Disclosure of Interests: Allyson Egan: None declared, pasupathy Sivasothy: None declared, Robin Gore: None declared, Marcos Martinez Del-Pero: None declared, Caroline Owen: None declared, Lisa Willcocks: None declared, Rona Smith: None declared, Stella Burns: None declared, David Jayne Grant/research support from: ChemoCentryx, GSK, Roche/Genentech, Sanofi-Genzyme, Consultant of: Astra-Zeneca, ChemoCentryx, GSK, InflaRx, Takeda, Insmed, Chugai, Boehringer-Ingelheim