

Background: The reason why some spondyloarthritis (SA) patients fail to respond to TNF inhibitors (TNF-i) remains unclear. Recently, it has been shown in cancer immunotherapy that the therapeutic response may be strongly altered by co-medication with drugs interfering with the gut microbiota, such as antibiotics, proton pump inhibitors (PPI), non-steroidal anti-inflammatory drugs (NSAIDs), psychotropic or antidiabetic drugs.
Objectives: Considering the potential role of the gut microbiota in the pathophysiology of SA as in the therapeutic response, the aim was to study the influence of co-medications known to interfere with the microbiota with the therapeutic response to TNF-i in SA patients.
Methods: We retrospectively reviewed the charts of all patients treated in our department with a first TNF-i from 2009 to 2018. Data collected were demographic information, HLA-B27 status, disease characteristics… Patients were classified as responder (R) or non-responder (NR) according to the BASDAI (< 40/100) value at M6 or to the clinician judgment (when BASDAI was not available). Regarding co-medications, we collected all drugs known to interfere with the gut microbiota that were administered 1 month before and during the first 3 months of the TNF-i treatment. We only considered drugs given to more than 5% of patients. Quantitative data were expressed as mean ± standard deviation, and qualitative variables as percentages. Univariate and multivariate analyses were performed to evaluate the relationship between co-medications and TNF-i. All analyses were computed on STATA 13.1 software with a statistically significant threshold of 0.05.
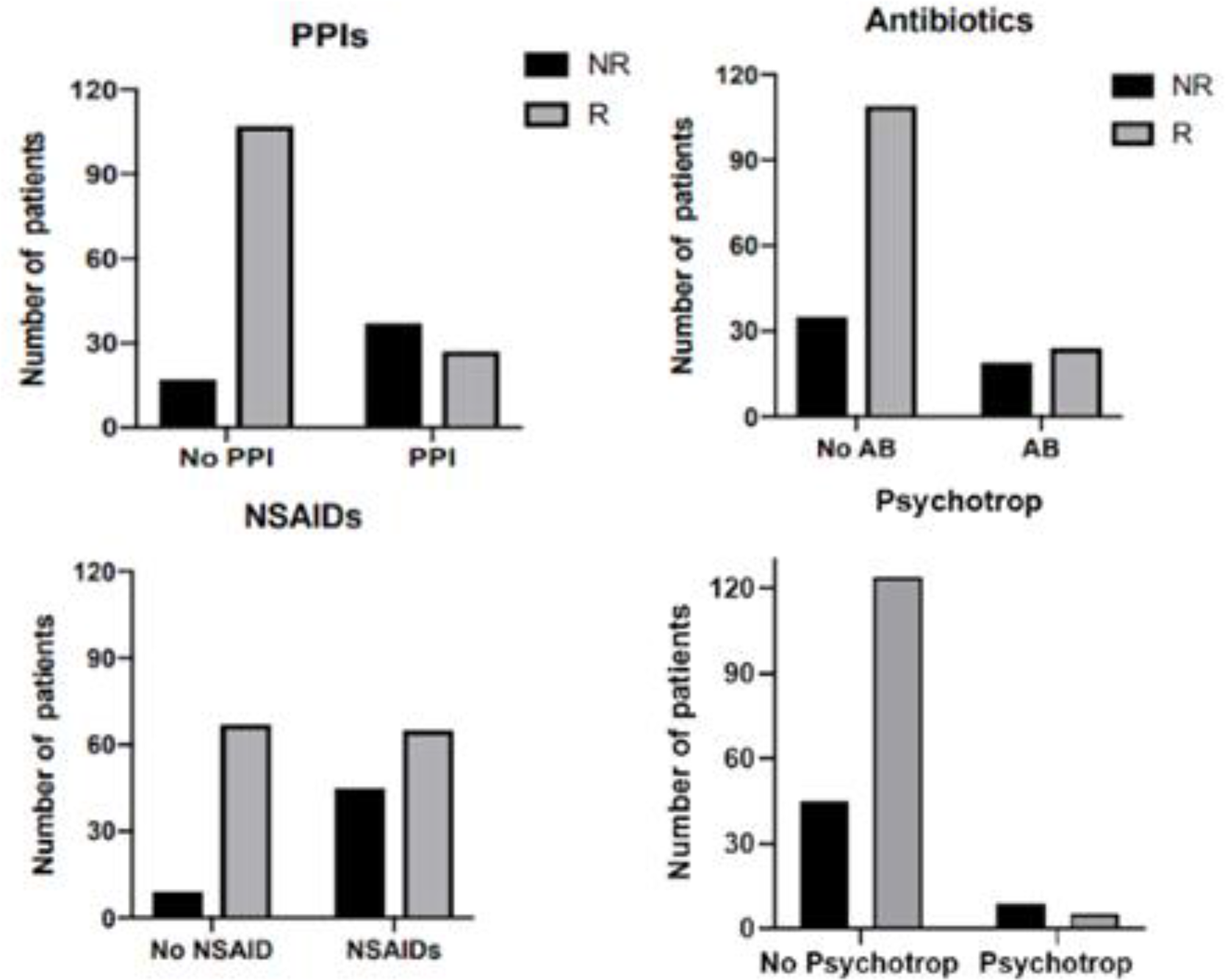
Results: We included 188 patients suffering from ankylosing spondylitis (n=89) or peripheral SA (n=99). They were 68 women and 120 men, mean aged 46.6 ± 13; 53% were B27 positive. TNF-i were infliximab (19%), etanercept (44%), adalimumab (34%) golimumab (2%), certolizumab (1%), combined with MTX in 51 patients. 135 patients (72%) were R and 53 (28%) NR. In univariate analysis, 59.1% of patients who received NSAIDs were R, compared to 88.2% of patients not treated with NSAIDs (p< 0.0001); 42.2% of patients receiving PPIs were R compared to 86.3% of patients PPI free (p< 0.0001); 55.8% of patients who were given antibiotics were R, compared to 75.7% of patients who did not (p=0,02); 27.8% of patients treated with psychotropic drugs were R, compared to 75.9% of patients not receiving such treatment (p< 0.0001) (Figure 1). Differences were not statistically significant for corticosteroids, MTX, angiotensin-converting enzyme inhibitors and statins. Although 91% of patients taken PPIs were also given NSAIDs, NSAIDs, PPIs and antibiotics intake were considered as independent factors associated with TNF-i failure in multivariate analysis.
Conclusion: Co-medication with NSAIDs, PPIs, antibiotics and psychotropic drugs were significantly associated with a decreased chance to respond to TNF-i. The hypothesis that this effect is due to their interference with the gut microbiota is only speculative but, regardless the reason of this interaction, clinician should be aware of the potential negative effect of these co-medication on TNF-i.
Disclosure of Interests: Maéva Masson: None declared, Marie Kostine: None declared, Thomas Barnetche: None declared, Marie-Elise Truchetet: None declared, Christophe Richez Consultant of: Abbvie, Amgen, Mylan, Pfizer, Sandoz and UCB., Thierry Schaeverbeke: None declared