

Background: Real-world data from electronic health records (EHR) allow examination of treatment patterns and clinical practice behaviors for psoriatic arthritis (PsA).
Objectives: To describe physician and patient characteristics, and treatment patterns of patients with PsA who initiated secukinumab and other biologics using data from the Columbus Repository.
Methods: EHR data from adult patients with PsA who were prescribed a new biologic therapy between January 2018 and March 2019 (index date) were included from the Columbus Repository, which collects clinical records from a network of US rheumatology providers. Demographics, disease characteristics, and treatment patterns, as well as physicians’ characteristics, were reported for patients who were prescribed secukinumab vs other biologics (abatacept, adalimumab, etanercept, certolizumab pegol, golimumab, infliximab, infliximab-dyyb, infliximab-abda, ustekinumab, and ixekizumab). Treatment groups were mutually exclusive and only the most recently prescribed biologic was represented. Categorical variables were summarized using frequency counts and percentages and continuous variables were presented using means and standard deviations.
Results: As of March 2019, 234 patients initiated secukinumab and 806 initiated other biologics for PsA treatment; 62 physicians prescribed biologics for PsA. Overall, 73% of physicians’ offices had a single provider contributing patients to the analysis, and 76% of physicians were located in the South US region. Secukinumab initiators were younger (55.2 vs 57.3 years), more likely to be male (44% vs 31%), and had higher BMI (34.0 vs 31.9 kg/m
2
) vs other biologic initiators. Almost all disease activity measures evaluated had a large proportion (> 80%) of missing data; among those with nonmissing data, secukinumab initiators had numerically higher mean (SD) RAPID3 score vs other biologic initiators (12.6 [6.5] vs 11.6 [7.1]). Overall, 70% of secukinumab initiators and 48% of other biologic initiators were biologic experienced (
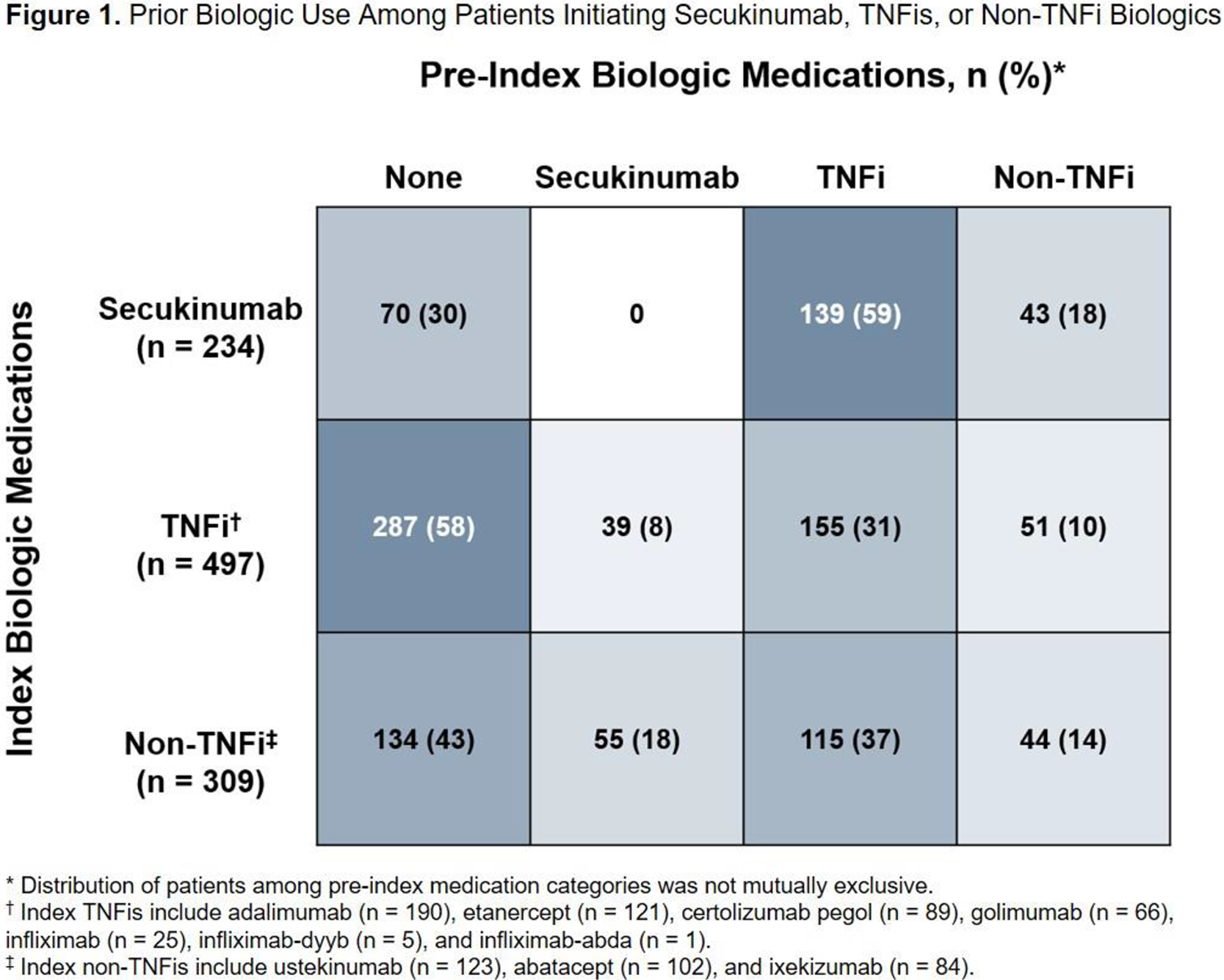
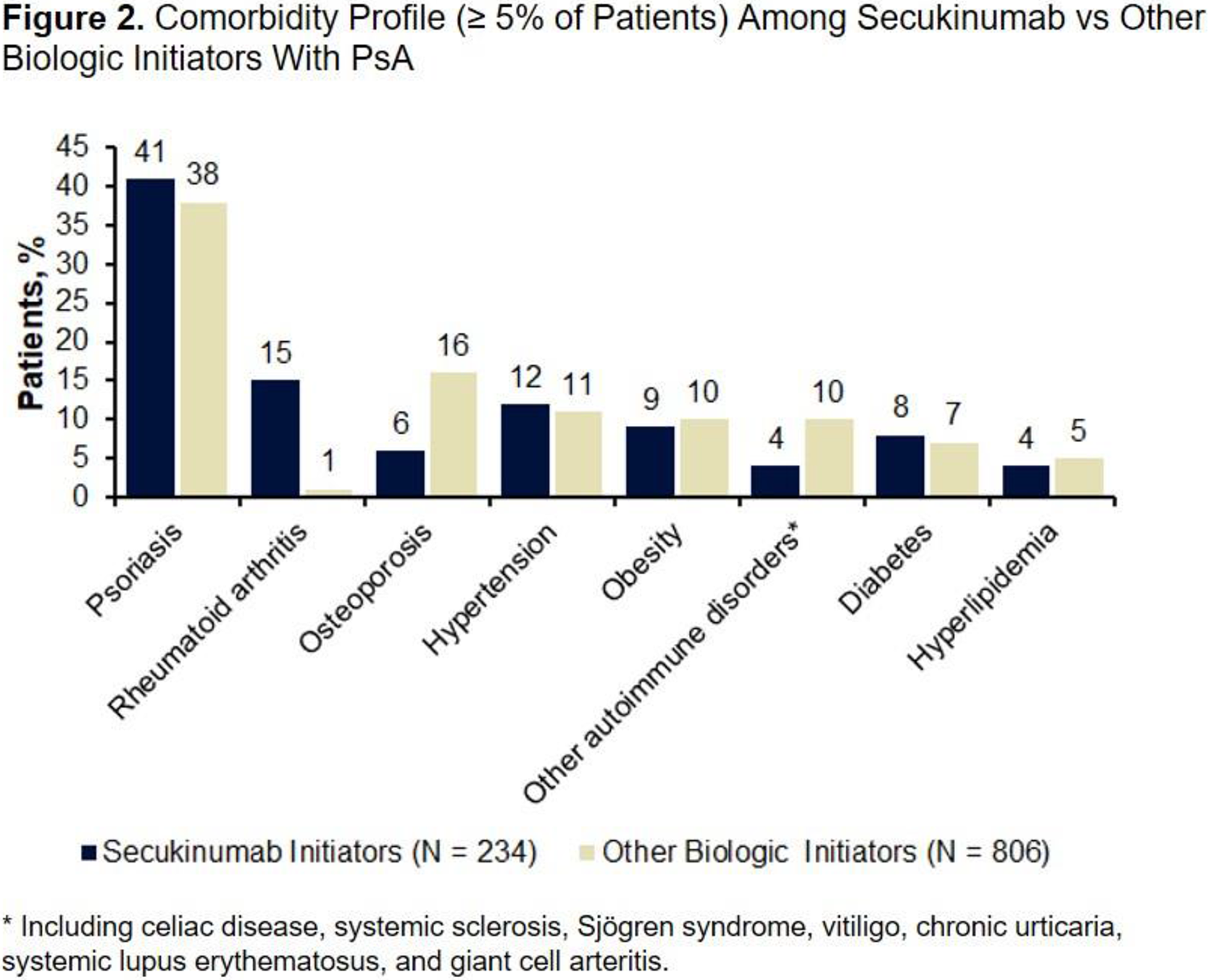
Treatment Patterns Among Patients With PsA at the Index Date
| Secukinumab
| Other Biologic
| SMD* | P Value | |
|---|---|---|---|---|
| Reason for discontinuing prior biologic treatment, n (%) | N = 164 | N = 385 | 0.20 | 0.67 |
| No longer required | 64 (39) | 136 (35) | ||
| Lack of efficacy | 28 (17) | 75 (19) | ||
| Cost or administrative | 5 (3) | 10 (3) | ||
| Side effects | 5 (3) | 9 (2) | ||
| Lack of tolerability | 0 | 3 (1) | ||
| Patient fear of side effects | 1 (1) | 0 | ||
| Other | 25 (15) | 63 (16) | ||
| Missing | 36 (22) | 89 (23) | ||
| Prior medication use, n (%) | ||||
| NSAIDs | 109 (47) | 365 (45) | 0.03 | 0.78 |
| Opioids | 89 (38) | 252 (31) | 0.14 | 0.06 |
| Steroids | 68 (29) | 265 (33) | 0.08 | 0.31 |
| DMARDs | ||||
| Methotrexate | 83 (35) | 340 (42) | 0.14 | 0.08 |
| Sulfasalazine | 25 (11) | 93 (12) | 0.03 | 0.81 |
| Apremilast | 53 (23) | 104 (13) | 0.26 | < 0.01 |
| Tofacitinib | 10 (4) | 36 (4) | 0.01 | 1.00 |
| No. of prior biologics, mean (SD) | 0.95 (0.82) | 0.62 (0.77) | 0.41 | < 0.01 |
SMD, standardized mean difference.
* Comparisons with SMD > 0.1 were suggestive of clinically relevant differences.
Conclusion: Secukinumab initiators with PsA were more likely to be male and biologic experienced, have a higher BMI and higher RAPID3 scores indicative of more active disease vs those initiating other biologics. Additional structured and unstructured elements may need to be captured on EHR platforms to gain clarity on disease activity and treatment decisions.
Acknowledgments: This study was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ. Support for third-party writing assistance for this abstract, furnished by Kheng Bekdache, PhD, of Health Interactions, Inc, was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Disclosure of Interests: Howard Busch Speakers bureau: AbbVie, Amgen, Crescendo, Exagen, Genentech, Mallinckrodt, Novartis, Primus, Sanofi/Regeneron, and UCB, Jeffrey Curtis Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, UCB, Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, UCB, Peter Hur Employee of: Novartis Pharmaceuticals Corporation