

Background: Patients with psoriatic arthritis (PsA) have skin manifestations that negatively affect their quality of life. Netakimab (NTK) is a humanized anti-interleukin 17A antibody approved for the treatment of moderate-to-severe plaque psoriasis.
Objectives: To evaluate the efficacy of NTK on PsA skin manifestations. This abstract presents 24-week data from the ongoing phase 3 PATERA study (NCT03598751).
Methods: PATERA is an international, multicenter, double-blind, placebo (PBO)-controlled study. 194 eligible adult patients with psoriatic arthritis (CASPAR, 2006), with inadequate response to csDMARD or one TNFi, were randomly assigned (1:1) to receive NTK 120 mg or placebo (PBO) subcutaneously at Week (Wk) 0, 1, 2, 4, 6, 8, 10, 14, 18, 22. 84 patients from PBO arm, failed to achieve ACR20 (20% improvement in American College of Rheumatology criteria) at Wk 16, were switched to NTK. Endpoints for assessment of skin involvement included PASI the proportion of patients achieving 75%, 90%, and 100% improvement in Psoriasis Area and Severity Index score (PASI75, PASI90 and PASI100, respectively).
Results: 184 patients (NTK arm, N=94; PBO arm, N=90) had PASI>0 at baseline (BL). Demographics and BL characteristics were balanced between treatment arms (
BL characteristics (for patients with PASI>0 at BL)
| Arm | NTK (N=94) | PBO (N=90) |
|---|---|---|
| Age (years) * | 44.0 (11.82) | 42.9 (12.14) |
| Male, n (%) | 51 (54.26) | 48 (53.33) |
| PsA duration, mo * | 63.3 (73.81) | 68.1 (79.79) |
| PASI * | 12.27 (11.31) | 10.35 (9.80) |
* mean (standard deviation); mo=months, PASI=Psoriasis Area and Severity Index
Percentage (%) change from baseline in PASI total score (mean (SD))
| NTK (N=76) | PBO (N=72) | |
|---|---|---|
| Week 4* | -61.1 (36.04) | -8.6 (31.99) |
| Week 8* | -74.2 (35.36) | -8.4 (37.57) |
| Week 16* | -80.8 (49.38) | -2.3 (61.06) |
| Week 24* | -87.5 (32.83) | -4.4 (63.48) |
Data are presented for patients with BL BSA ≥ 3%; *p<0.0001 vs placebo
Percentage of patients with PASI75/90/100 at Wk 24 (in patients with BL BSA≥3)
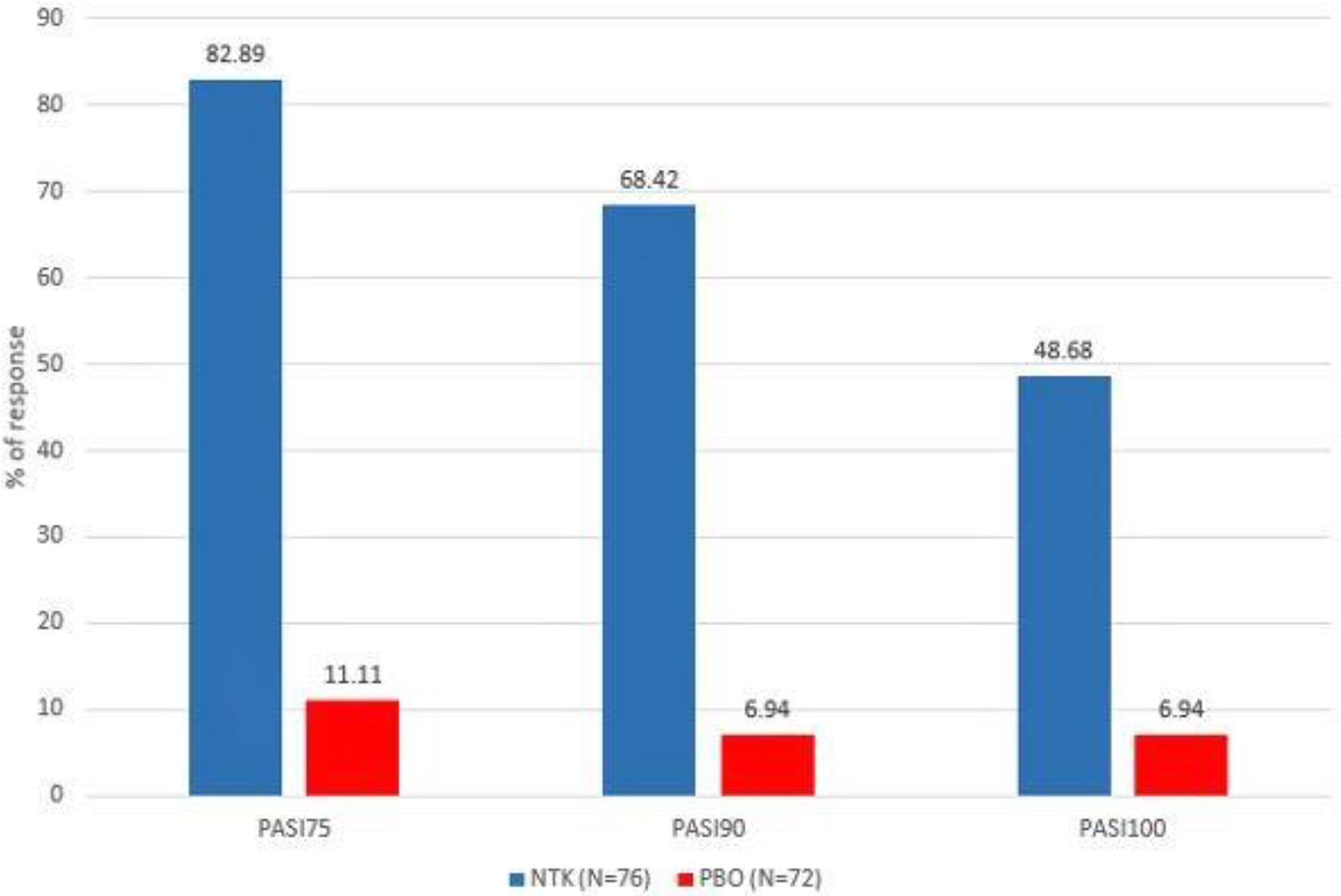
Conclusion: 24-week treatment with NTK at the dose of 120 mg resulted in significant improvement in skin manifestations in PsA patients: more than half of the patients with BSA≥3 at BL achieved complete skin clearance.
Acknowledgments: This study was sponsored by JSC BIOCAD.
Disclosure of Interests: Tatiana Korotaeva Consultant of: Pfizer, MSD, Novartis, AbbVie, Celgene, JSC BIOCAD, Janssen, UCB, Lilly and Novartis-Sandoz, Speakers bureau: Pfizer, MSD, Novartis, AbbVie, Celgene, JSC BIOCAD, Janssen, UCB, Lilly and Novartis-Sandoz, Inna Gaydukova Grant/research support from: JSC BIOCAD, Speakers bureau: Pfizer, Novartis, AbbVie, JSC BIOCAD, Сelgene, MSD, Sanofi, V Mazurov: None declared, Aleksey Samtsov Grant/research support from: JSC BIOCAD, Novartis, Eli Lilly, Johnson&Johnson, Celgene, Glenmark, Galderma, Sanofi, Vladislav Khayrutdinov Grant/research support from: Akrikhin, Alkoy, Belupo, JSC BIOCAD, Bosnaliejk, Verteks, Glenmark, Elfa, Leo Pharma, MSD, Novartis, Pfizer, Sun Pharma, Sanofi, Celgene, Pharmtec, AbbVie, Eli Lilly, Jadran, Janssen, Andrey Bakulev Grant/research support from: AbbVie, Eli Lilly, Pfizer, UCB, MSD, Novartis, Galderma, Celgene, Leo Pharma and Johnson&Johnson, JSC BIOCAD, Consultant of: Novartis, Celgene and Johnson&Johnson, Speakers bureau: AbbVie, Eli Lilly, Galderma, UCB, Novartis, Celgene and Johnson&Johnson, Muza Kokhan Grant/research support from: AbbVie, Eli Lilly, Pfizer, UCB, MSD, Novartis, Galderma, Celgene, Leo Pharma and Johnson&Johnson, JSC BIOCAD, Consultant of: Novartis, Celgene and Johnson&Johnson, Speakers bureau: AbbVie, Eli Lilly, Galderma, UCB, Novartis, Celgene and Johnson&Johnson, Alena Kundzer: None declared, Nikolaj Soroka Grant/research support from: JSC BIOCAD, Ekaterina Dokukina Employee of: JSC BIOCAD, Anna Eremeeva Employee of: JSC BIOCAD