

Background: Psoriatic arthritis (PsA) is associated with multiple manifestations, resulting in reduced health-related quality of life (HR-QoL) of patients (pts). Netakimab (NTK) is a humanized anti-IL17A antibody approved for the treatment of moderate-to-severe plaque psoriasis.
Objectives: To assess the impact of NTK on patient-reported outcomes (PROs) in active PsA pts, based on data of 24-week (wk) data from the ongoing PATERA study (NCT03598751).
Methods: PATERA is an international double-blind, placebo-controlled clinical study. 194 eligible adult pts with PsA (CASPAR, 2006), with inadequate response to csDMARD or one TNFi, were randomized (1:1) to receive NTK 120 mg or placebo (PBO) at Wk 0, 1, 2, 4, 6, 8, 10, 14, 18, 22. Pts from PBO arm failed to achieve ACR20 (20% improvement of the American College of Rheumatology criteria) by Wk 16 were switched to NTK. PROs included changes from baseline (BL) in Work Productivity and Activity Impairment General Health (WPAI GH), 36-item Short Form Health Survey (SF-36), European Quality of Life Questionnaire (EQ-5D-5L), Dermatology Quality of Life Index (DLQI), Health assessment questionnaire disability index (HAQ-DI).
Results: BL demographics and PsA characteristics were similar between treatment arms (
BL demographics and PsA characteristics
| Arm | NTK (N=97 ) | PBO (N=97 ) |
|---|---|---|
| Age (years) * | 44.0 (11.7) | 43.1 (11.9) |
| Male, n (%) | 52 (53.6) | 50 (51.6) |
| PsA duration, mo * | 63.1 (73.1) | 68.2 (77.5) |
| HAQ-DI * | 1.15 (0.6) | 1.21 (0.6) |
| DLQI * | 14.8 (6.5) | 13.9 (7.3) |
| SF36 PCS * | 32.39 (9.5) | 31.10 (8.9) |
| SF36 MCS * | 45.29 (10.7) | 46.04 (11.5) |
* mean (standard deviation), N=number of pts, mo=months, PsA=psoriatic arthritis, HAQ-DI=Health assessment questionnaire disability index, DLQI=Dermatology Quality of Life Index, SF36=36-item Short Form Health Survey, MCS=Mental Component Summary, PCS=Physical Component Summary
WPAI change from BL at wk 24 (mean±standard deviation)
| Parameter | NTK | PBO |
|---|---|---|
| Absenteeism (%) | -8.7±29.1
| -9.1±31.8
|
| Presenteeism (%) | -22.1 ±22.1
| -1.0±26.5
|
| Overall work impairment (%) | -18.6±21.8
| 0.6±26.7
|
| Activity impairment (%) | -25.5±25.2
| -5.4±29.1
|
N=number of pts in the analysis category
Improvement in EQ-5D categories
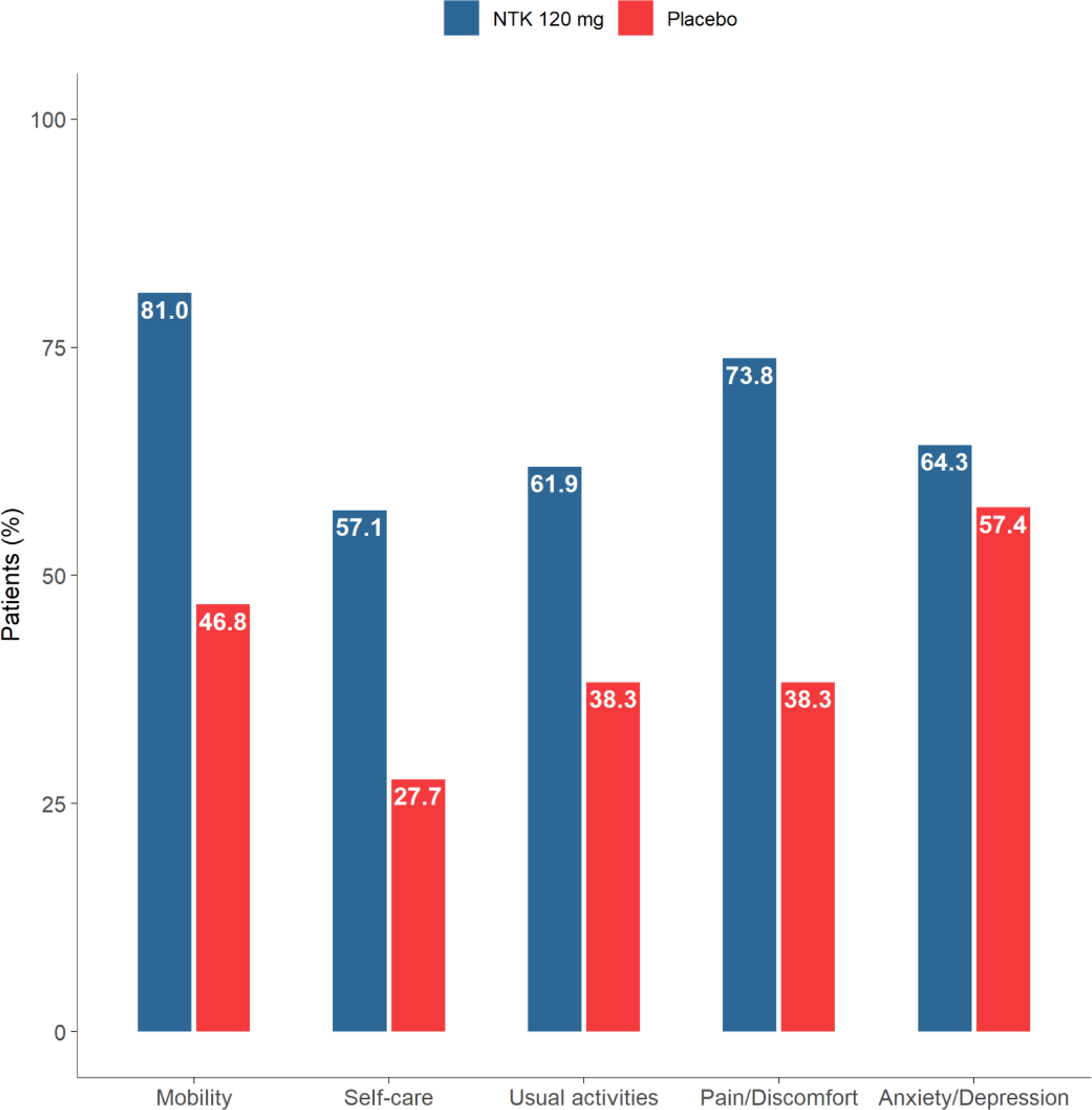
Conclusion: NTK demonstrated rapid improvement in QoL, work productivity and physical function in pts with PsA.
Acknowledgments: This study was sponsored by JSC BIOCAD.
Disclosure of Interests: Tatiana Korotaeva Consultant of: Pfizer, MSD, Novartis, AbbVie, Celgene, JSC BIOCAD, Janssen, UCB, Lilly and Novartis-Sandoz, Speakers bureau: Pfizer, MSD, Novartis, AbbVie, Celgene, JSC BIOCAD, Janssen, UCB, Lilly and Novartis-Sandoz, Inna Gaydukova Grant/research support from: JSC BIOCAD, Speakers bureau: Pfizer, Novartis, AbbVie, JSC BIOCAD, Сelgene, MSD, Sanofi, V Mazurov: None declared, Aleksey Samtsov Grant/research support from: JSC BIOCAD, Novartis, Eli Lilly, Johnson&Johnson, Celgene, Glenmark, Galderma, Sanofi, Vladislav Khayrutdinov Grant/research support from: Akrikhin, Alkoy, Belupo, JSC BIOCAD, Bosnaliejk, Verteks, Glenmark, Elfa, Leo Pharma, MSD, Novartis, Pfizer, Sun Pharma, Sanofi, Celgene, Pharmtec, AbbVie, Eli Lilly, Jadran, Janssen, Andrey Bakulev Grant/research support from: AbbVie, Eli Lilly, Pfizer, UCB, MSD, Novartis, Galderma, Celgene, Leo Pharma and Johnson&Johnson, JSC BIOCAD, Consultant of: Novartis, Celgene and Johnson&Johnson, Speakers bureau: AbbVie, Eli Lilly, Galderma, UCB, Novartis, Celgene and Johnson&Johnson, Muza Kokhan Grant/research support from: AbbVie, Eli Lilly, Pfizer, UCB, MSD, Novartis, Galderma, Celgene, Leo Pharma and Johnson&Johnson, JSC BIOCAD, Consultant of: Novartis, Celgene and Johnson&Johnson, Speakers bureau: AbbVie, Eli Lilly, Galderma, UCB, Novartis, Celgene and Johnson&Johnson, Alena Kundzer: None declared, Nikolaj Soroka Grant/research support from: JSC BIOCAD, Ekaterina Dokukina Employee of: JSC BIOCAD, Anna Eremeeva Employee of: JSC BIOCAD