

Background: Patients (pts) with psoriatic arthritis (PsA) experience broad systemic symptoms including pain, fatigue, depression, sleep disturbance, poor physical function, and diminished social participation.
Objectives: DISCOVER 1 is a Phase 3 trial (NCT03162796) evaluating the efficacy and safety of guselkumab (GUS), an anti-interleukin 23 inhibitor that binds to the p19-subunit of IL-23, in pts with active PsA. PROMIS-29 (Patient-Reported Outcomes Measurement Information System-29), a validated generic health instrument, 1 assessed the treatment effect of GUS on symptoms in pts with PsA.
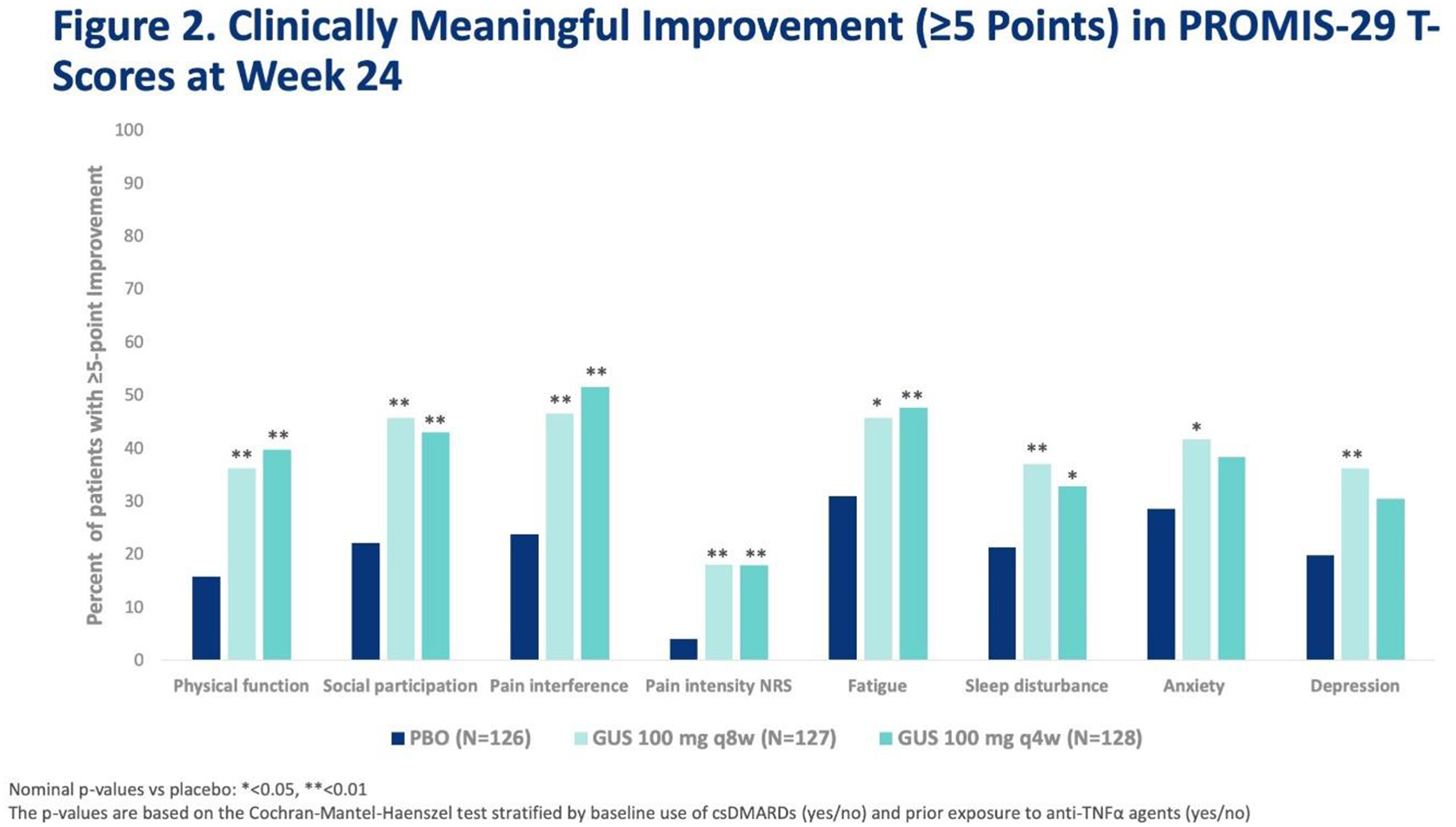
Methods: Pts with active PsA despite nonbiologic DMARDs were enrolled, and ~30% of pts could have previously received ≤2 TNFi. Pts were randomized (1:1:1) to subcutaneous GUS 100 mg at Week 0 (W0), W4 then q8W (n=127), GUS 100 mg q4W (n=128), or PBO (n=126). Concomitant stable use of select csDMARDs, oral steroids, and NSAIDs was allowed. PROMIS-29 consists of 7 domains (Depression, Anxiety, Physical Function, Pain Interference, Fatigue, Sleep Disturbance, and Social Participation) and a pain intensity 0-10 numeric rating scale (NRS). The raw score of each domain is converted into a standardized T-score with a mean of 50 (general population mean) and a standard deviation (SD) of 10. Higher PROMIS scores represent more of the concept being measured. A >= 5-point improvement (1/2 SD of T-score) is defined as clinically meaningful. 1
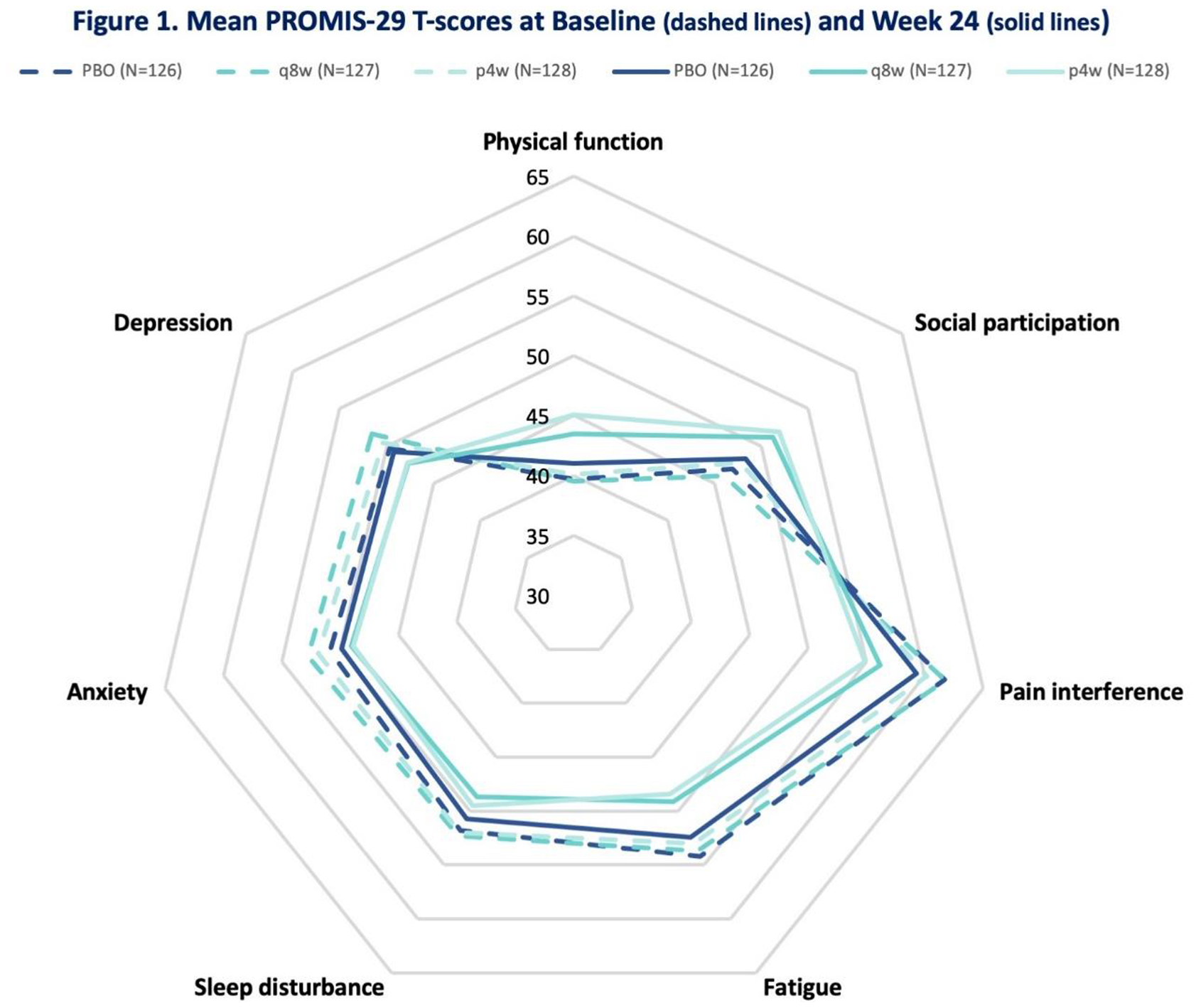
Results: At baseline, mean PROMIS-29 T-scores for physical function, social participation, sleep disturbance, pain, and fatigue were worse than the general US population. At W24, GUS q8W-treated pts achieved greater improvements from baseline in all PROMIS-29 domains vs PBO (p<0.05) (Table and
Conclusion: Active PsA pts treated with GUS achieved clinically meaningful reduction in symptoms and improvement in physical function and social participation vs PBO at W24.
REFERENCES:
[1]http://www.healthmeasures.net/score-and-interpret/interpret-scores/meaningful-change/165-meaningful-change
PROMIS-29 Domain T-Scores Least Square (LS) Mean Change from Baseline
| LS Mean Change from Baseline | |||
|---|---|---|---|
| PBO | GUS q8W | GUS q4W | |
| Anxiety | -1.37 | -3.23* | -2.92 |
| Depression | -0.85 | -3.4** | -2.67* |
| Fatigue | -1.86 | -4.79** | -5.08** |
| Pain interference | -2.30 | -5.49** | -5.69** |
| Physical function | 1.34 | 3.89** | 5.05** |
| Sleep disturbance | -1.17 | -3.48** | -2.46 |
| Social participation | 1.45 | 4.90** | 4.52** |
| Pain intensity | -0.56 | -1.98** | -2.32** |
Nominal p-values vs placebo: *<0.05, **<0.01
Acknowledgments: None
Disclosure of Interests: Ana-Maria Orbai Grant/research support from: Abbvie, Eli Lilly and Company, Celgene, Novartis, Janssen, Horizon, Consultant of: Eli Lilly; Janssen; Novartis; Pfizer; UCB. Ana-Maria Orbai was a private consultant or advisor for Sun Pharmaceutical Industries, Inc, not in her capacity as a Johns Hopkins faculty member and was not compensated for this service., Laura C Coates: None declared, Atul Deodhar Grant/research support from: AbbVie, Eli Lilly, GSK, Novartis, Pfizer, UCB, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myer Squibb (BMS), Eli Lilly, GSK, Janssen, Novartis, Pfizer, UCB, Speakers bureau: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myer Squibb (BMS), Eli Lilly, GSK, Janssen, Novartis, Pfizer, UCB, Philip Helliwell: None declared, Christopher T. Ritchlin Grant/research support from: UCB Pharma, AbbVie, Amgen, Consultant of: UCB Pharma, Amgen, AbbVie, Lilly, Pfizer, Novartis, Gilead, Janssen, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Elizabeth C Hsia Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Xie L Xu Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Shihong Sheng Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Bei Zhou Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Chenglong Han Employee of: Janssen Research & Development, LLC