

Background: The efficacy of the interleukin (IL)-23 subunit p19 inhibitor guselkumab (GUS) for psoriatic arthritis (PsA) has recently been demonstrated in two Phase 3 trials (DISCOVER-1 & -2) but has not been evaluated versus existing targeted therapies for PsA.
Objectives: To compare GUS to targeted therapies for PsA through network meta-analysis (NMA).
Methods: A systematic literature review was performed to identify PsA randomized controlled trials from 2000 to 2018. Bayesian NMAs were performed to compare treatments on American College of Rheumatology (ACR) 20/50/70 response, Psoriasis Area Severity Index (PASI) 75/90/100 response, Health Assessment Questionnaire Disability Index (HAQ-DI) score, resolution of enthesitis (RoE), resolution of dactylitis (RoD), adverse events (AEs) and serious adverse events (SAEs). Analyses used random effects models that adjusted for placebo response via meta-regression on baseline risk when feasible. Results are summarized by ranking treatments according to median absolute probabilities of response derived from NMAs.
Results: Twenty-six Phase 3 studies were included in the quantitative synthesis. Studies were placebo-controlled up to 24 weeks and evaluated 13 targeted therapies for PsA. Absolute probabilities are reported for PASI 90 & ACR 20 responses according to Figure 1, and a forest plot of relative risks versus placebo for AEs is reported according to Figure 2 . For ACR 20 response, GUS 100 mg every 4 weeks (Q4W) and every 8 weeks (Q8W) ranked 5th and 8th out of 20 interventions and were comparable to IL-17A inhibitor (IL-17Ai) and most tumor necrosis factor inhibitor (TNFi) agents. Similar findings were observed for ACR 50 and 70 responses. For PASI 90 response, GUS Q4W and Q8W ranked 1st and 2nd out of 15 interventions and were highly likely to provide a greater benefit than most other agents. Similar findings were observed for PASI 75 and 100 responses. For HAQ-DI score, GUS Q4W and Q8W ranked 6th and 10th out of 20 interventions and were comparable to IL-17Ai and most TNFi agents. For RoE, GUS Q4W and Q8W ranked 8th and 6th out of 13 interventions and were comparable to IL-17Ai and TNFi agents. For RoD, GUS Q4W and Q8W ranked 8th and 9th out of 13 interventions and were comparable to most IL-17Ai and TNFi agents. For AEs, GUS Q4W and Q8W ranked 3rd and 2nd out of 19 interventions and were comparable to IL-17Ai and TNFi agents. Likewise, for SAEs, GUS Q4W and Q8W ranked 4th and 5th out of 20 interventions and were comparable to IL-17Ai and TNFi agents. Analyses that controlled for previous exposure to biologics or assessed outcomes at alternative timepoints were broadly consistent with primary analysis results.
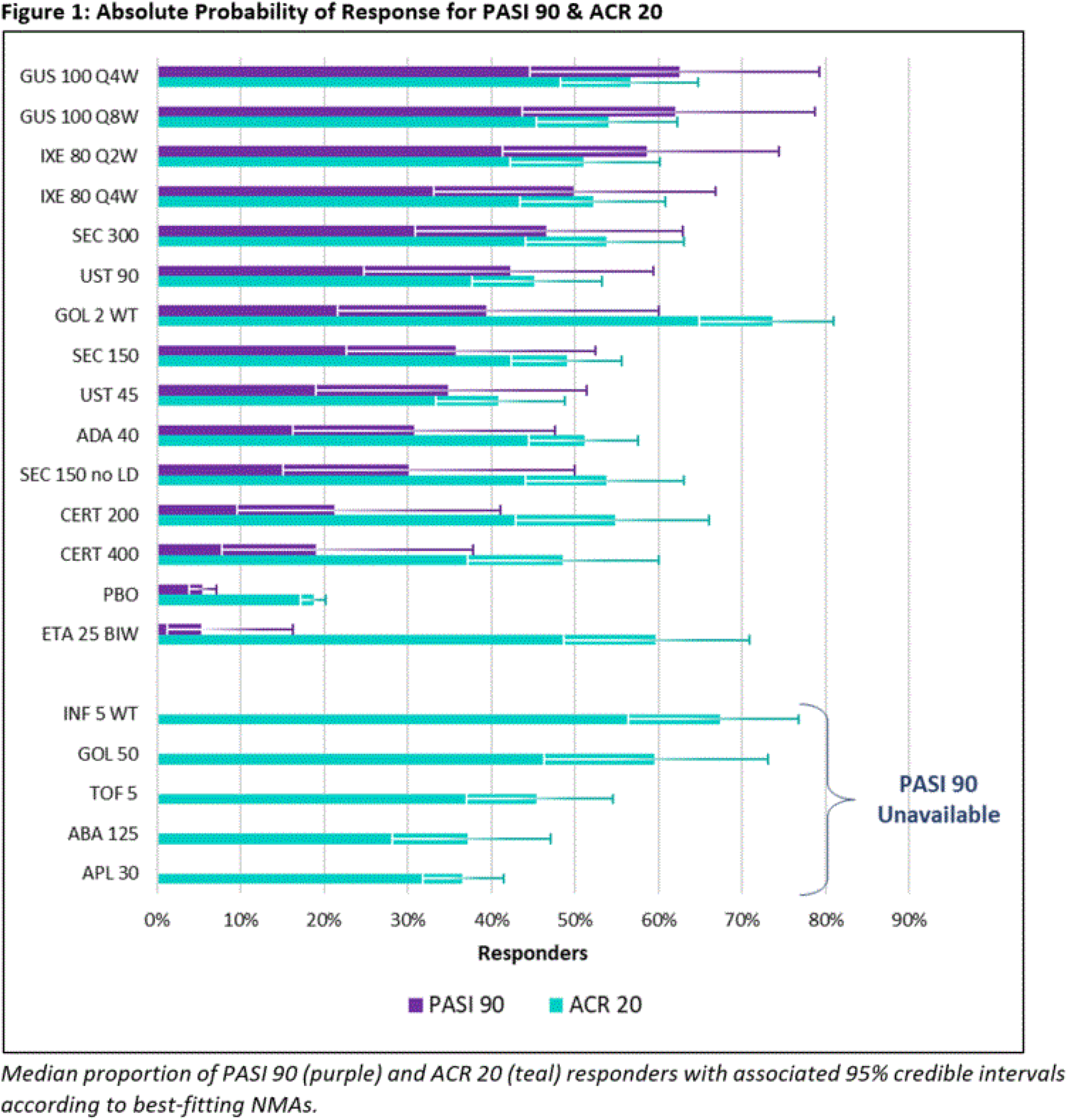
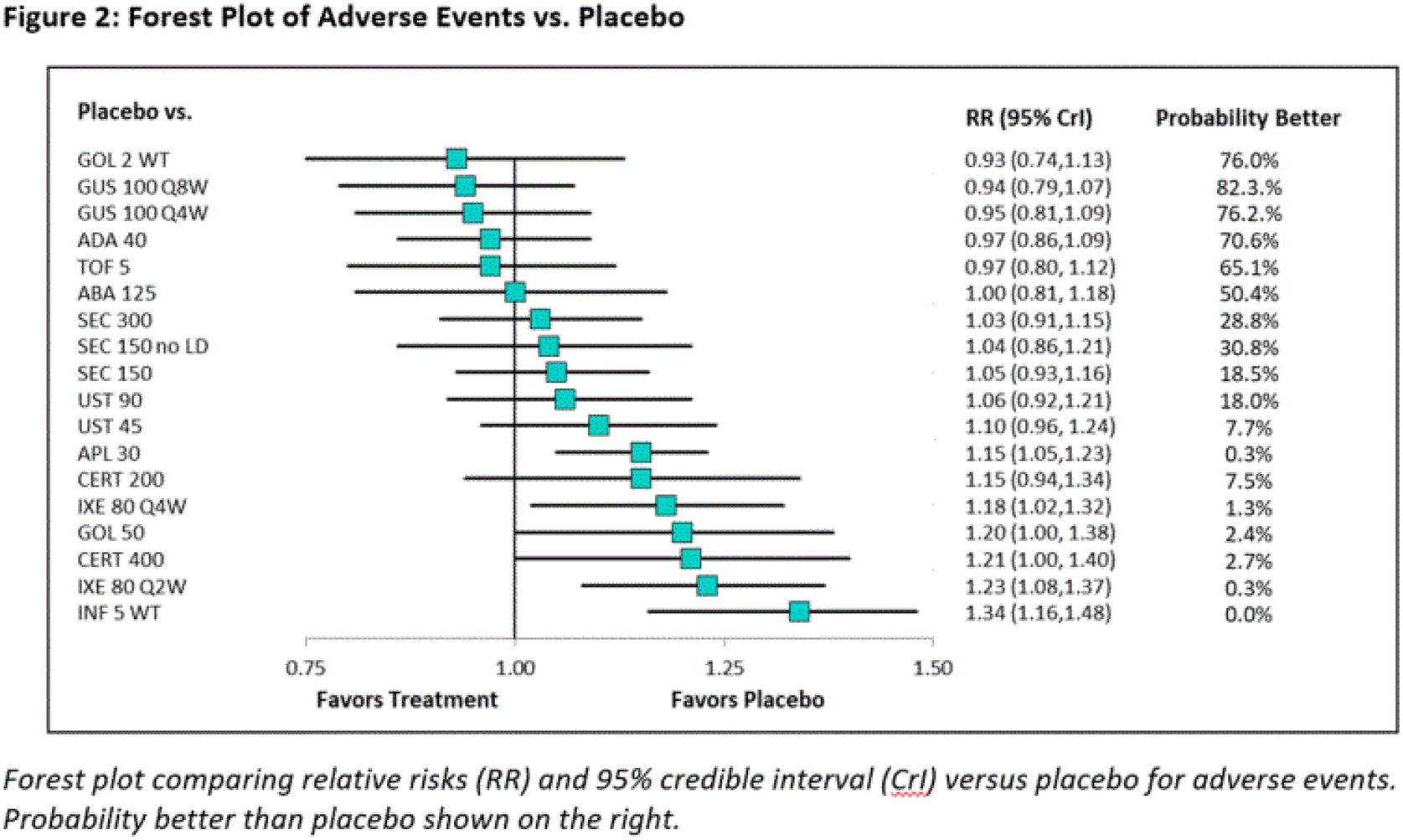
Conclusion: NMA results indicate that GUS is comparable to most targeted PsA treatments for improvement in arthritis, soft tissue damage, physical function, and safety outcomes. For PASI outcomes, GUS is highly likely to provide a greater benefit than other targeted PsA treatments.
Disclosure of Interests: Iain McInnes Grant/research support from: Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, and UCB, Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, Novartis, Pfizer, and UCB, Philip J Mease Grant/research support from: Abbott, Amgen, Biogen Idec, BMS, Celgene Corporation, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, UCB – grant/research support, Consultant of: Abbott, Amgen, Biogen Idec, BMS, Celgene Corporation, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, UCB – consultant, Speakers bureau: Abbott, Amgen, Biogen Idec, BMS, Eli Lilly, Genentech, Janssen, Pfizer, UCB – speakers bureau, Kiefer Eaton Shareholder of: Test Pharma, Consultant of: Janssen, Agata Schubert Employee of: Janssen-Cilag, Steve Peterson Employee of: Janssen Research & Development, LLC, Tim Disher Consultant of: Janssen, Wim Noel Employee of: Janssen Pharmaceuticals NV, Hassan Fareen Employee of: Janssen, Chetan Karyekar Shareholder of: Johnson & Johnson, Consultant of: Janssen, Employee of: Janssen Global Services, LLC. Previously, Novartis, Bristol-Myers Squibb, and Abbott Labs., Suzy Van Sanden Employee of: Janssen, Christopher T. Ritchlin Grant/research support from: UCB Pharma, AbbVie, Amgen, Consultant of: UCB Pharma, Amgen, AbbVie, Lilly, Pfizer, Novartis, Gilead, Janssen, Wolf-Henning Boehncke Grant/research support from: Janssen Research & Development, LLC, Consultant of: Janssen