

Background: Acute Charcot neuroarthropathy (CN) of the foot is a rare and severe complication of peripheral neuropathy leading to joint destruction. Usual treatment rely on standard pressure offloading and no pharmacological treatment is available. Inflammation and increased osteoclastic activity via receptor activator of nuclear factor (RANK) ligand are major features of acute CN.
Objectives: To assess clinical, metabolic and radiographic effect of denosumab, a fully human monoclonal antibody against RANK ligand, in acute CN.
Methods: In this open study, we included all consecutive patients with acute CN treated with denosumab 60 mg in our mixed rheumatology/diabetes clinic dedicated to diabetic foot. Diagnosis of acute CN was based on clinical presentation and supported by biology, radiography, magnetic resonance imaging (MRI). Baseline and follow-up assessment included clinical examination and emission tomography–computed tomography (PET-CT).
Results: Seven patients with acute CN were treated with denosumab between 2017 and 2019 (age from 43 to 70 years). Five were diabetic. All patients received denosumab, because of failure of standard pressure offloading, with evolving joint destruction of midfoot. CN evolves since a median of 6 months (2 to 20) at denosumab initiation. All patients clinically improved after denosumab injection (table). After a mean follow-up of 16 months, only 1/7 patients had a new flare. In the 4 patients with available follow-up X-ray, structural damage remained stable. In all 3 patients with available PET-CT evolution, the maximum standardized uptake lean value (SUL max) decreased.
Baseline characteristics and follow up treatment
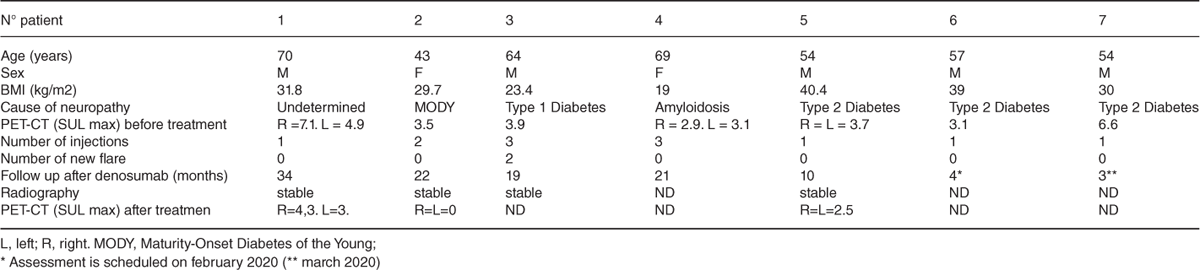
Three patients were retreated, with a mean interval of 6 months: One patients because of persistent clinical and biological inflammation (CRP 17 mg/L), one because of relapse due to intensive walking, and one due to an associated osteoporosis.
No adverse event and hypocalcemia was observed.
Conclusion: One to three injection of denosumab 60 mg was efficient in preventing flare and further bone destruction in a 16 months medium follow up. These results justify the conduction of a randomized control study to assess the efficacy of denosumab as the first-line pharmacological therapy in acute CN.
REFERENCES:
[1]Molines L and al. Charcot’s foot: Newest findings on its pathophysiology, diagnosis and treatment. Diabetes Metab. Sept 2010;36(4):251-5.
[2]Mabilleau G, and al. Increased osteoclastic activity in acute Charcot’s osteoarthropathy: the role of receptor activator of nuclear factor-kappaB ligand. Diabetologia. juin 2008;51(6):1035-40.
Disclosure of Interests: None declared
Disclosure of Interests: Sandrine Carvès: None declared, Julien Henry: None declared, Murielle BOURGEON GHITTORI: None declared, Rakiba Belkhir: None declared, Gaetane Nocturne: None declared, Florent Besson: None declared, Guillaume Cluzel: None declared, Maud Creze: None declared, Raphaèle Seror Consultant of: BMS UCB Pfizer Roche, Xavier Mariette Consultant of: BMS, Gilead, Medimmune, Novartis, Pfizer, Servier, UCB