

Background: Anti-TNFα agents are useful in uveitis (1-5). Certolizumab pegol (CZP) differs from other anti-TNFα agents due to its limited placental transfer.
Objectives: To assess efficacy and safety of CZP in women with uveitis during pregnancy.
Methods: Multicenter study of women with uveitis under CZP during pregnancy and their neonates.
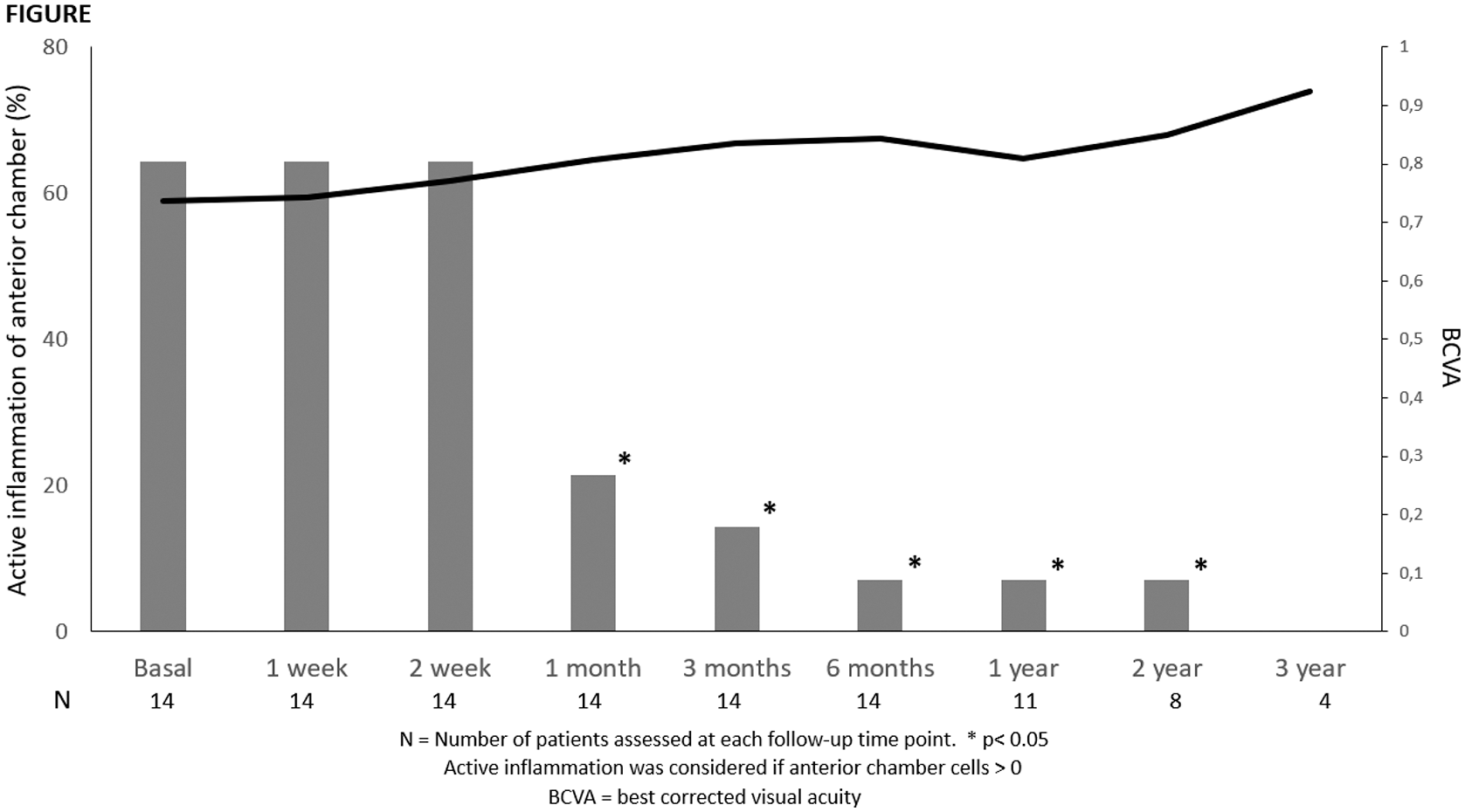
Results: 14 women (23 eyes); mean age 34.3±5.5 yrs (
| Age | Underlying disease | Immunosuppressants before CZP | Combined treatment | |
|---|---|---|---|---|
| 1 | 34 | SpA | MTX, AZA, ADA | AZA |
| 2 | 37 | SpA | MTX, AZA, IFX, ADA, GOLI | |
| 3 | 39 | SpA | AZA, ADA | AZA |
| 4 | 46 | SpA | CyA, ETN, ADA, IFX, GOLI | |
| 5 | 32 | SpA | SSZ, ADA | SSZ |
| 6 | 36 | SpA | MTX, HCQ, ADA | |
| 7 | 40 | SpA | MTX, LFN, HCQ, IFX, ADA, GOLI | HCQ |
| 8 | 31 | Idiopathic | MTX, MMF, CyA, ADA | |
| 9 | 33 | Idiopathic | MTX, AZA, ADA, ETN | |
| 10 | 32 | RA | MTX | AZA |
| 11 | 23 | Vogt-Koyanagi-Harada | AZA, ADA | AZA |
| 12 | 36 | Juvenil Idiopathic Arthritis | ADA | |
| 13 | 32 | Punctate inner choroidopathy | ADA | |
| 14 | 29 | Behcet | CyA, IFX, ADA |
Conclusion: CZP seems to be effective and safe in female patients with uveitis during pregnancy and neonates.
REFERENCES:
[1]Llorenç V et al. Certolizumab Pegol, a New Anti-TNF-α in the Armamentarium against Ocular Inflammation. Ocul Immunol Inflamm. 2016;24(2):167-72. doi: 10.3109/09273948.2014.967779
[2]Urruticoechea-Arana A et al. Efficacy and safety of biological therapy compared to synthetic immunomodulatory drugs or placebo in the treatment of Behçet’s disease associated uveitis: a systematic review. Rheumatol Int. 2019 Jan;39(1):47-58. doi: 10.1007/s00296-018-4193-z
[3]Martín-Varillas JL et al. Successful Optimization of Adalimumab Therapy in Refractory Uveitis Due to Behçet’s Disease Ophthalmology. 2018 Sep;125(9):1444-1451. doi: 10.1016/j.ophtha.2018.02.020
[4]Santos-Gómez M et al. The effect of biologic therapy different from infliximab or adalimumab in patients with refractory uveitis due to Behçet’s disease: results of a multicentre open-label study. Clin Exp Rheumatol. 2016. Sep-Oct;34(6 Suppl 102): S34-S40
[5]Calvo-Río V et al. Golimumab in refractory uveitis related to spondyloarthritis. Multicenter study of 15 patients.Semin Arthritis Rheum. 2016 Aug;46(1):95-101. doi: 10.1016/j.semarthrit.2016.03.002
Disclosure of Interests: D. Prieto-Peña: None declared, Monica Calderón-Goercke: None declared, Alfredo Adan: None declared, Lillian Chamorro-López: None declared, Olga Maiz: None declared, JR De Dios-Jiménez Aberásturi: None declared, Raul Veroz Gonzalez: None declared, Soledad Blanco: None declared, José M Santos: None declared, Francisco Navarro: None declared, Adela Gallego: None declared, Senen González-Suárez: None declared, Arantxa Conesa: None declared, Andrea García-Valle: None declared, Miguel Cordero-Coma: None declared, Nieves Pardiñas-Barón: None declared, Rosalía Demetrio-Pablo: None declared, Vanesa Calvo-Río Grant/research support from: MSD and Roche, Speakers bureau: AbbVie, Lilly, Celgene, Grünenthal, UCB Pharma, Victor Manuel Mora-Cuesta: None declared, Santos Castañeda: None declared, J. Luis Hernández: None declared, Miguel A González-Gay Grant/research support from: Pfizer, Abbvie, MSD, Speakers bureau: Pfizer, Abbvie, MSD, Ricardo Blanco Grant/research support from: AbbVie, MSD, Roche, Consultant of: Abbvie, Eli Lilly, Pfizer, Roche, Bristol-Myers, Janssen, UCB Pharma and MSD, Speakers bureau: Abbvie, Eli Lilly, Pfizer, Roche, Bristol-Myers, Janssen, UCB Pharma. MSD