

Background: Early diagnosis and rapid initiation of DMARDs following a treat-to-target approach have made remission a realizable goal for many with RA. Yet, some patients are unable to sustain remission over time.
Objectives: To describe longitudinal patterns of remission and identify predictors of sustained vs transient remission in real-world early RA patients.
Methods: Data were from the Canadian Early Arthritis Cohort (CATCH), a prospective study of early RA patients (symptoms < 1 year) treated in rheumatology clinics across Canada from 2007- 2019. The sample was limited to patients with active disease at enrolment who later reached remission (SDAI<=3.3) and were followed for 12-24 months thereafter. Patients were classified as in sustained remission (Pattern 1) or transient remission with transient remission patients divided into those who transitioned from REM to LDA only (Pattern 2) and those who transitioned from REM to MDA or HDA (Pattern 3), over FU. Multi-adjusted multinomial regression was used to identify predictors of transient remission patterns.
Results: The study included 1,419 (46%) CATCH participants that reached remission. At enrolment, most (70%) were female, mean(sd) SDAI was high (27(15)) and 92% were treated with csDMARDs. Only 47% remained in sustained remission by 12-months and, only 40% by 24 months (Pattern 1) (
Adjusted Multinomial Regression Results of Predictors of Transient Remission Patterns over 24-Month Follow Up
| Pattern 2 vs, Pattern 1
| Pattern 3 vs. Pattern 1
|
|
|---|---|---|
| Age | 1.01 (1.00, 1.02) | 1.01 (0.99, 1.02) |
| Women vs Men | 1.78 (1.33, 2.39 ) | 1.63 (1.09, 2.44 ) |
| Current smoker | 1.57 (1.09, 2.28 ) | 1.53 (0.95, 2.47) |
| RDCI at baseline | 1.11 (0.99, 1.25) | 1.30 (1.13, 1.50 ) |
| Seropositive | 1.38 (1.03, 1.85 ) | 1.21 (0.81, 1.80) |
| MTX first 3 months | 1.18 (0.85, 1.63) | 0.76 (0.51, 1.12) |
| Time to remission (months) | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.02) |
| Treatment reduction after REM vs. No Change | 1.33 (0.96, 1.86) | 1.01 (0.99, 1.02) |
Pattern 1: Sustained REM
Pattern 2: Transient REM: Transitions to LDA only
Pattern 3: Transient REM: Transitions to MDA/HDA
RDCI: Rheumatic Disease Comorbidity Index (range 0-9)
Treatment reduction: Change from biologic or JAK to csDMARD(s) OR reduction in number of csDMARDs OR change from MTX +/- csDMARDs to non-MTX csDMARD
Distribution of Disease Activity States over 12-24 After First Achieving SDAI REM
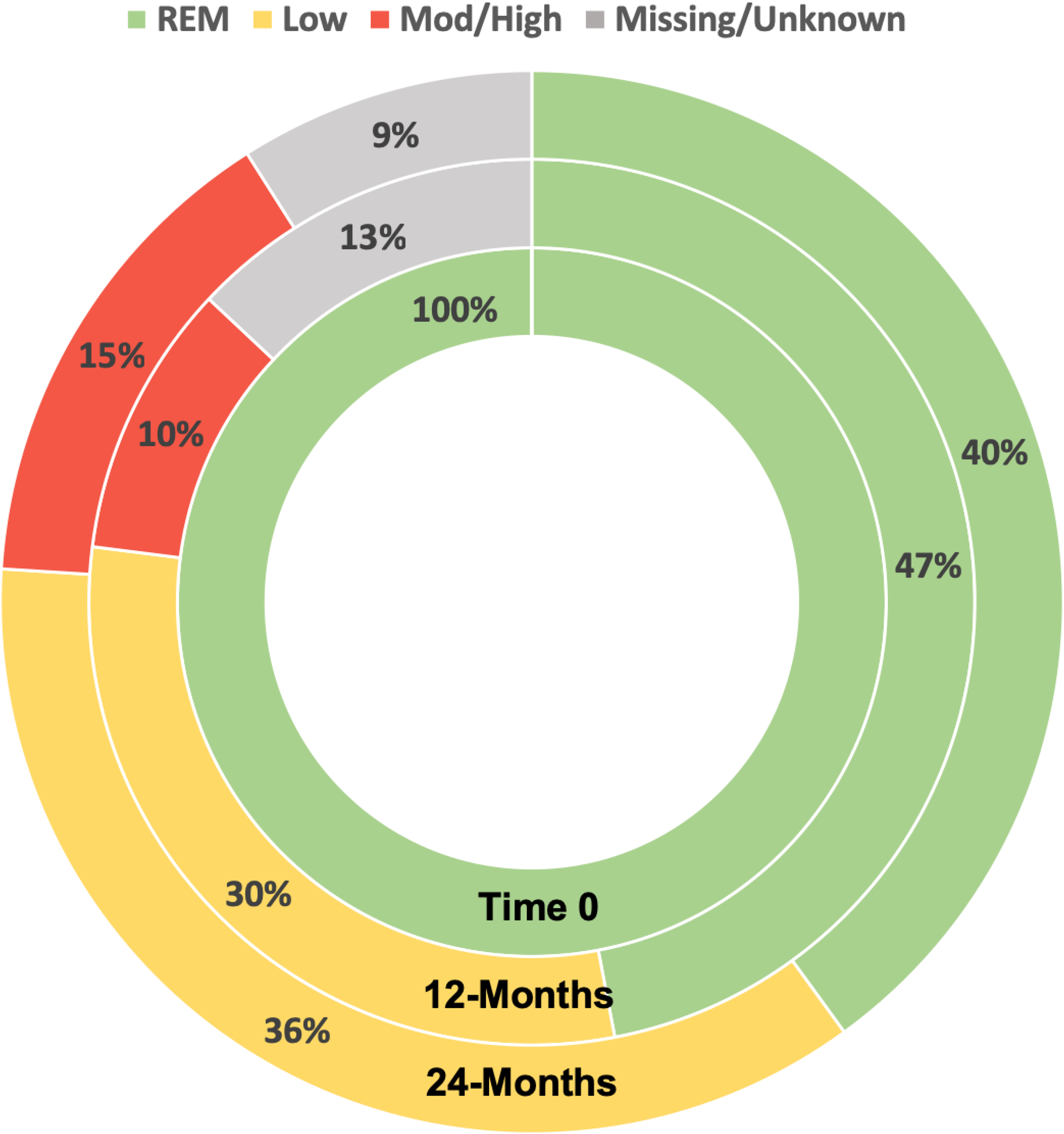
Conclusion: Results of this large longitudinal analysis of real-world data suggests that < 50% of patients that reach remission sustain remission for 12-24months. Closer monitoring of patients with prognostic indicators for transient remission and additional research focusing on why remission is lost may help improve the rates of sustained remission.
REFERENCES:
[1]Ajeganova S, Huizinga T. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis. 2017;9(10):249-62.
Disclosure of Interests: Orit Schieir: None declared, Glen Hazlewood: None declared, Susan J. Bartlett Consultant of: Pfizer, UCB, Lilly, Novartis, Merck, Janssen, Abbvie, Speakers bureau: Pfizer, UCB, Lilly, Novartis, Merck, Janssen, Abbvie, Marie-France Valois: None declared, Louis Bessette Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Roche, Sanofi, UCB Pharma, Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Roche, Sanofi, UCB Pharma, Speakers bureau: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Sanofi, Gilles Boire Grant/research support from: Merck Canada (Registry of biologices, Improvement of comorbidity surveillance)
Amgen Canada (CATCH, clinical nurse)
Abbvie (CATCH, clinical nurse)
Pfizer (CATCH, Registry of biologics, Clinical nurse)
Hoffman-LaRoche (CATCH)
UCB Canada (CATCH, Clinical nurse)
BMS (CATCH, Clinical nurse, Observational Study Protocol IM101664. SEROPOSITIVITY IN A LARGE CANADIAN OBSERVATIONAL COHORT)
Janssen (CATCH)
Celgene (Clinical nurse)
Eli Lilly (Registry of biologics, Clinical nurse), Consultant of: Eli Lilly, Janssen, Novartis, Pfizer, Speakers bureau: Merck, BMS, Pfizer, Carol Hitchon Grant/research support from: UCB Canada; Pfizer Canada, Edward Keystone Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, F. Hoffmann-La Roche Inc, Gilead, Janssen Inc, Lilly Pharmaceuticals, Pfizer Pharmaceuticals, Sanofi-Aventis, Consultant of: AbbVie, Amgen, AstraZeneca Pharma, Biotest, Bristol-Myers Squibb Company, Celltrion, Crescendo Bioscience, F. Hoffmann-La Roche Inc, Genentech Inc, Gilead, Janssen Inc, Lilly Pharmaceuticals, Merck, Pfizer Pharmaceuticals, Sandoz, UCB., Speakers bureau: Amgen, AbbVie, Bristol-Myers Squibb Canada, F. Hoffmann-La Roche Inc., Janssen Inc., Merck, Pfizer Pharmaceuticals, Sanofi Genzyme, UCB, Janet Pope Grant/research support from: AbbVie, Bristol-Myers Squibb, Eli Lilly & Company, Merck, Roche, Seattle Genetics, UCB, Consultant of: AbbVie, Actelion, Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eicos Sciences, Eli Lilly & Company, Emerald, Gilead Sciences, Inc., Janssen, Merck, Novartis, Pfizer, Roche, Sandoz, Sanofi, UCB, Speakers bureau: UCB, Carter Thorne Consultant of: Abbvie, Centocor, Janssen, Lilly, Medexus/Medac, Pfizer, Speakers bureau: Medexus/Medac, Diane Tin: None declared, Vivian Bykerk: None declared