

Background: Infliximab is still a widely used biologic agent in treatment of rheumatoid arthritis (RA). Because infliximab is expensive and can have adverse events, identification of factors that predict an adequate response to this treatment has been investigated.
Objectives: In this study, we investigated the association between rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) status and the discontinuation of infliximab therapy due to adverse events or insufficient response in bio-naïve patients with RA.
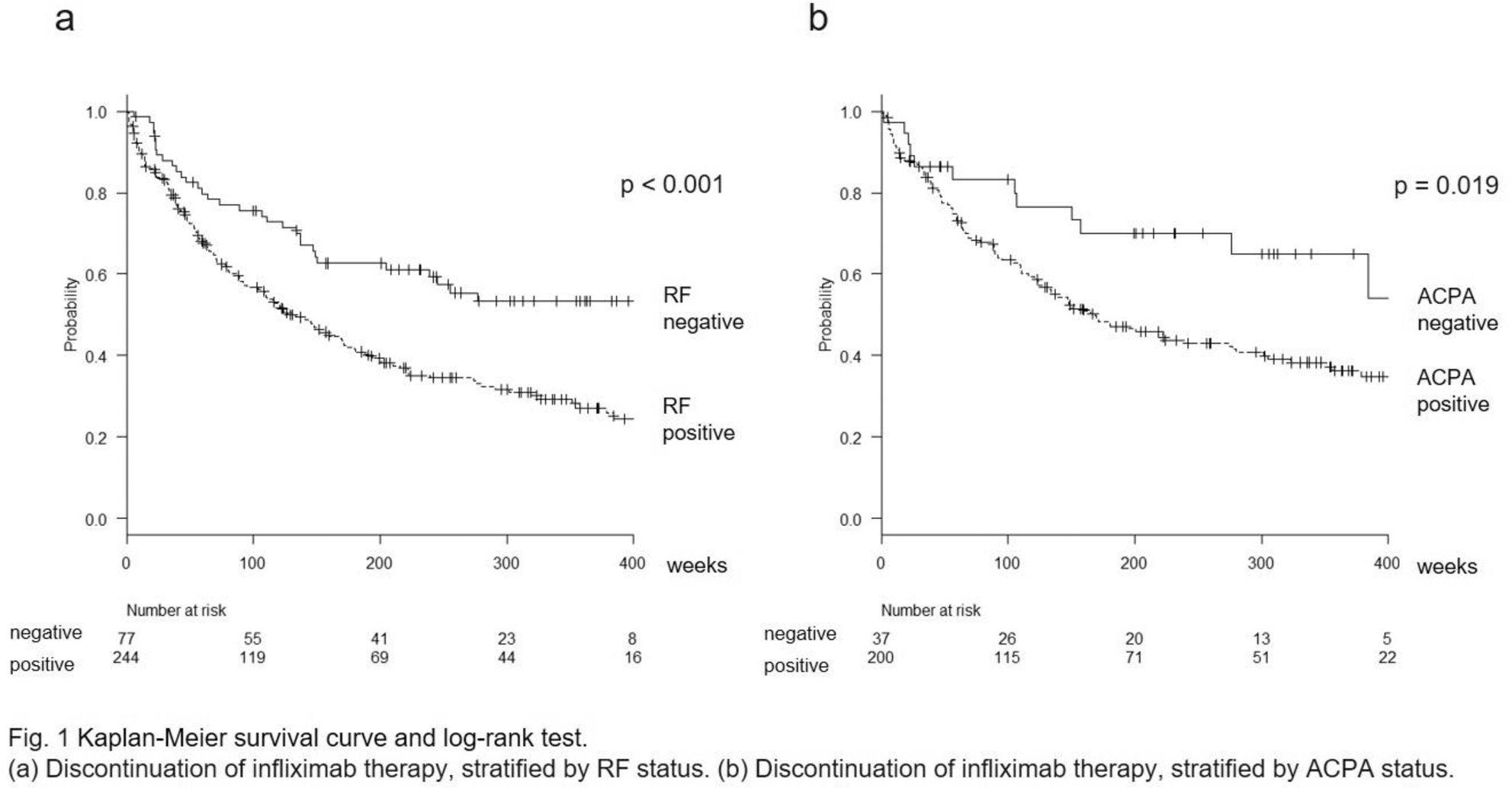
Methods: This study included patients enrolled in the Tsurumai Biologic Communication Registry in Japan. A crude comparison of infliximab discontinuation between seropositive and seronegative patients was using Kaplan-Meier analysis and log-rank test. We evaluated the associations between the specified baseline characteristics and discontinuation of infliximab therapy using Cox proportional hazard regression. We could not perform simultaneous assessments of the impact of RF and ACPA seropositivity on clinical efficacy becasue of collinearity.
Results: Baseline characteristics of the patients included in this study are shown in
Characteristics of RA patients at baseline by RF and ACPA status
| RF (n = 344; | ACPA (n = 250; | |||||
|---|---|---|---|---|---|---|
| 985 patient-years) | 824 patient-years) | |||||
| RF | RF | ACPA | ACPA | |||
| positive | negative | positive | negative | |||
| (n = 263) | (n = 81) | P † | (n = 211) | (n = 39) | P † | |
| Age, years (SD) | 55.7 (12.3) | 54.6 (13.9) | 0.48 | 55.4 (12.3) | 49.7 (14.3) | 0.01 |
| Female, no. (%) | 205 (78.2) | 66 (81.5) | 0.64 | 170 (80.6) | 28 (71.8) | 0.28 |
| DAS28ESR (SD) | 5.50 (1.33) | 4.95 (1.51) | 0.005 | 5.54 (1.28) | 4.61 (1.82) | 0.0005 |
| Stage I+II/III+IV, no. (%) | 81/174 (31.8/68.2) | 25/50 (33.3/66.7) | 0.78 | 61/139 (30.5/69.5) | 15/20 (42.9/57.1) | 0.17 |
| Class I+II/III+IV, no. (%) | 155/102 (60.3/39.7) | 52/22 (70.3/29.7) | 0.14 | 126/72 (63.6/36.4) | 23/12 (65.7/34.3) | 0.85 |
| Current MTX treatment, % | 100 | 100 | 1 | 100 | 100 | 1 |
| MTX dose, mg/week (SD) ‡ | 7.56 (2.16) | 7.80 (2.22) | 0.4 | 7.82 (2.20) | 7.31 (2.66) | 0.22 |
| Current PSL treatment, no. (%) | 141 (68.1) | 37 (56.1) | 0.077 | 128 (67.4) | 19 (55.9) | 0.24 |
| PSL dose, mg/day (SD) ‡ | 3.98 (3.91) | 2.70 (2.74) | 0.01 | 3.73 (3.78) | 2.63 (2.85) | 0.11 |
| BMI, kg/m 2 (SD) | 22.6 (3.88) | 21.3 (4.22) | 0.1 | 22.0 (4.10) | 22.5 (3.33) | 0.68 |
Data are presented as mean, unless otherwise stated. SD: standard deviation
† Chi-square test for categorical variables and t-test for continuous variables.
‡ MTX dose and PSL dose were mean value in patients with concomitant MTX and PSL treatment, respectively.
Cox proportional hazard regression for infliximab therapy due to adverse event and insufficient response
| Model including RF status (n = 226) | Model including ACPA status (n = 182) | ||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | P | Variable | HR (95% CI) | P |
| RF positive | 1.99 (1.25-3.18) | 0.0037 | ACPA positive | 2.73 (1.24-6.02) | 0.012 |
| Age at baseline | 0.99 (0.98-1.01) | 0.43 | Age at baseline | 0.99 (0.98-1.01) | 0.36 |
| Sex (referent: male) | 1.21 (0.76-1.94) | 0.41 | Sex (referent: male) | 0.99 (0.60-1.62) | 0.96 |
| Prednisolone use | 1.03 (0.71-1.49) | 0.85 | Prednisolone use | 1.02 (0.67-1.56) | 0.92 |
| Stage III + IV (referent: I + II) | 1.01 (0.99-1.03) | 0.17 | Stage III + IV (referent: I + II) | 1.01 (0.98-1.03) | 0.54 |
| Class III + IV (referent: I + II) | 0.99 (0.98-1.02) | 0.73 | Class III + IV (referent: I + II) | 0.99 (0.97-1.01) | 0.55 |
| DAS28ESR at baseline | 0.95 (0.83-1.10) | 0.54 | DAS28ESR at baseline | 0.99 (0.84-1.18) | 0.97 |
Conclusion: RF and ACPA seropositivity in bio-naïve patients with RA correlated with a higher rate of infliximab discontinuation due to adverse events or ineffectiveness.
Yoshikazu Ogawa: None declared, Nobunori Takahashi Speakers bureau: AbbVie, Asahi Kasei, Astellas, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Janssen, Mitsubishi Tanabe, Ono, Pfizer, Takeda, and UCB Japan, Toshihisa Kojima Grant/research support from: Chugai, Eli Lilly, Astellas, Abbvie, and Novartis, Consultant of: AbbVie, Speakers bureau: AbbVie, Astellas, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eli Lilly, Janssen, Mitsubishi Tanabe, Pfizer, and Takeda, Naoki Ishiguro Grant/research support from: AbbVie, Asahi Kasei, Astellas, Chugai, Daiichi-Sankyo, Eisai, Kaken, Mitsubishi Tanabe, Otsuka, Pfizer, Takeda, and Zimmer Biomet, Consultant of: Ono, Speakers bureau: Astellas, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, and Taisho Toyama