

Background: Tofacitinib (TOF) is an orally administered Janus Kinase (JAK) inhibitor and is commonly used in rheumatoid arthritis. There is a heterogeneity among numbers reported from different continents about herpes zoster (HZ) incidence rate (1-3). However, data about HZ risk in our country, which stands like a bridge between Asia and Europe, is lacking.
Objectives: To assess the real-life incidence of herpes zoster in RA patients under tofacitinib.
Methods: We analyzed all patients who had at least 1 control visit under tofacitinib and registered to HURBIO database. We calculated incidence rate by dividing the number of patients with herpes zoster to total follow-up years, then multiplied by 100 (per 100 patient-years).
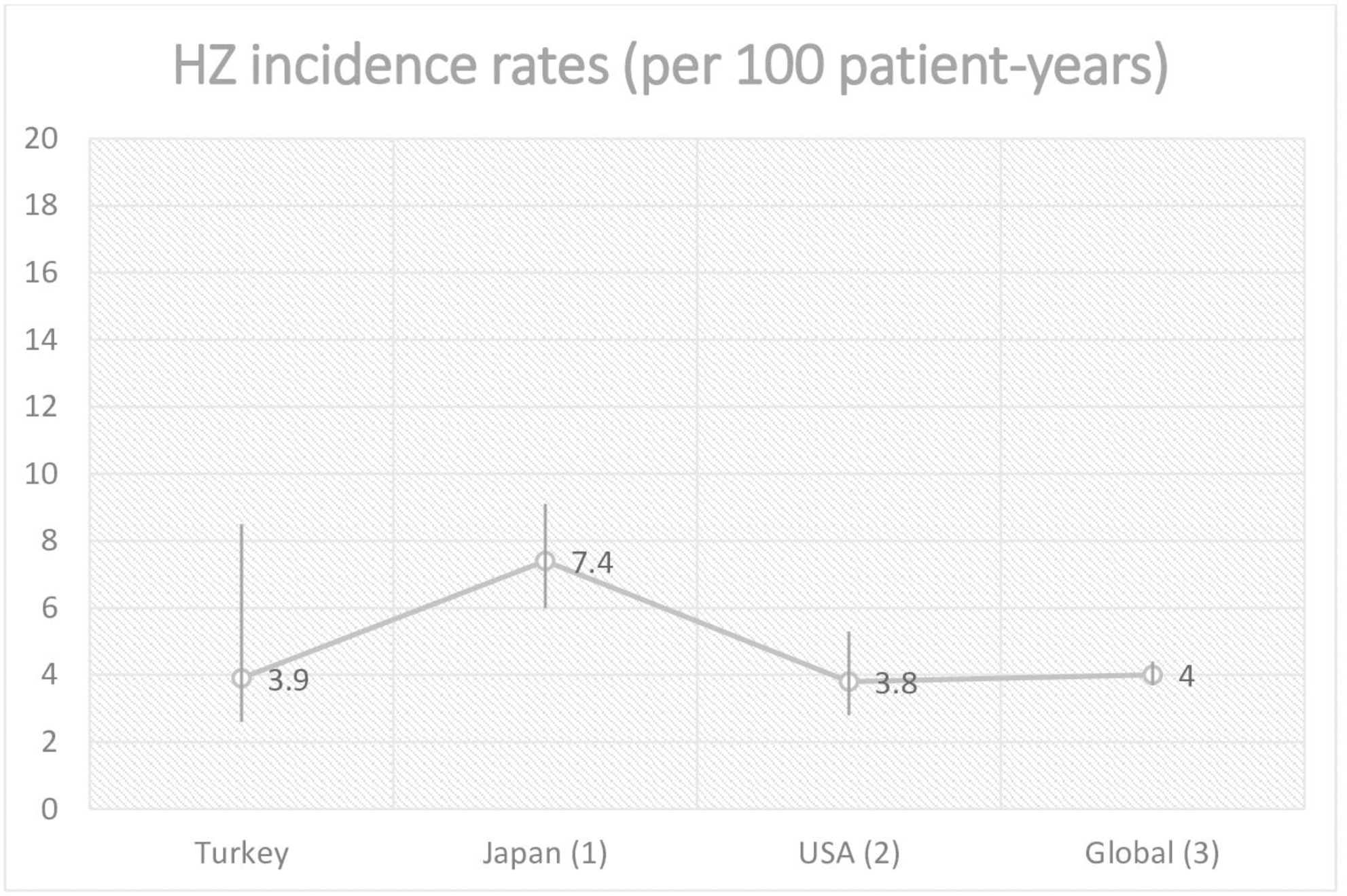
Results: A total of 204 (174 (85.4%) female) patients were recruited. Mean age was 53.2±12.5 years. Mean disease duration was 11.5±8.1 years. Rheumatoid factor and anti-CCP antibodies were positive in 135/198 (68.1 %) and 115/171 (67.2 %) patients, respectively. Median follow-up while receiving TOF was 11.6 (IQR:5.2-26.2) months. Combination with DMARDs was used in 83.3% of patients. 55.5% of patients was biologic-naive. Eleven (5.3%, incidence rate: 3.9 (2.3-8.5; % 95 CI) per 100 patient-years) patients had zona zoster. Ten of these patients was female, median age was 59 (IQR; 52-69) and 4 of them was older than 65 years-old. Rheumatoid factor was positive in 9 patients. Only 1 of these patients had diabetes. Median follow-up of these patients under TOF was 8.1(IQR: 6-25) months. Ten of these patients had concomitant DMARDs (9 hydroxycholoroquine, 4 methotrexate, 2 leflunomide; according to last follow-up visit) and 9 of them received concomitant steroids (med(IQR); 4 (1-8) mg- at equivalant methyl-prednisolon dose). Eight of them was biologic-naive. Tofacitinib was discontinued in 4 of these patients.
Conclusion: In this real-life data from Turkey, we found a HZ incidence rate similar to that reported from USA and global data; however, we found a lower incidence rate that reported from Japan (
Reported herpes zoster incidence rates across different countries (numbers in paranthesis indicate reference number)
REFERENCES:
[1]Winthrop KL, Curtis JR, Lindsey S, Tanaka Y, Yamaoka K, Valdez H, et al. Herpes Zoster and Tofacitinib: Clinical Outcomes and the Risk of Concomitant Therapy. Arthritis & rheumatology (Hoboken, NJ). 2017;69(10):1960-8.
[2]Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1843-7.
[3]Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther. 2016;18:34.
Disclosure of Interests: Emre Bilgin: None declared, Furkan Ceylan: None declared, Emine Duran: None declared, Ertugrul Cagri Bolek: None declared, Bayram Farisoğullari: None declared, Gözde Kübra Yardimci: None declared, Levent Kiliç: None declared, Ali Akdoğan: None declared, Omer Karadag: None declared, Şule Apraş Bilgen: None declared, Sedat Kiraz: None declared, Ali İhsan Ertenli: None declared, Umut Kalyoncu Consultant of: Abbvie, Amgen, Janssen, Lilly, Novartis, UCB