

Background: Secukinumab was the first anti-interleukin 17A monoclonal antibody treatment approved by the FDA for ankylosing spondylitis (AS). There is scarce information on the characteristics of secukinumab vs other biologic initiators with AS.
Objectives: To describe real-world physician and patient characteristics, and treatment patterns of secukinumab and tumor necrosis factor inhibitor (TNFi) initiators.
Methods: Electronic health records (EHR) data from adult patients with AS who initiated a biologic therapy between January 2018 and March 2019 (index date) were included from the Columbus Repository, a network capturing EHR data from 120 US rheumatology providers. Physician and patient characteristics, and treatment patterns were reported for patients who were prescribed secukinumab and TNFis (adalimumab, etanercept, certolizumab pegol, infliximab, infliximab-abda, and golimumab). Categorical variables were summarized using frequency counts and percentages and continuous variables were presented using means and standard deviations. Standardized mean differences and P values were used to compare treatment groups.
Results: As of March 2019, AS treatment data were available for 82 secukinumab initiators and 160 TNFi initiators. Regarding overall practice size, 33% of practices had a single physician, and 65% of physicians were located in the South US region. Secukinumab initiators were younger than TNFi initiators (47.4 vs 49.8 years) and had a similar prevalence of HLA-B27 positivity (≈ 55%;
Baseline Demographics and Disease Characteristics Among Patients With AS at the Index Date.
| . | ||||
|---|---|---|---|---|
| Characteristic | Secukinumab
| TNFi
| SMD* | P Value |
| Age, mean (SD), years | 47.4 (12.8) | 49.8 (14.6) | 0.17 | 0.21 |
| Female, n (%) | 43 (52) | 90 (56) | 0.08 | 0.57 |
| Race/ethnicity, n (%) | N = 65 | N = 129 | 0.20 | 0.66 |
| White | 52 (80) | 106 (82) | ||
| Hispanic | 8 (12) | 13 (10) | ||
| Black | 3 (5) | 8 (6) | ||
| Asian | 1 (2) | 2 (2) | ||
| Other | 1 (2) | 0 | ||
| Geographic distribution, n (%) | 0.37 | 0.05 | ||
| South | 49 (60) | 113 (71) | ||
| Midwest | 27 (33) | 28 (18) | ||
| West | 5 (6) | 16 (10) | ||
| Northeast | 1 (1) | 3 (2) | ||
| Health insurance, n (%) | N = 79 | N = 156 | 0.41 | 0.39 |
| Commercial | 57 (72) | 94 (60) | ||
| Medicare | 8 (10) | 33 (21) | ||
| Medicaid | 2 (3) | 4 (3) | ||
| Other | 12 (15) | 25 (16) | ||
| HLA-B27 positivity, n (%) [N] | 14 (54) [N = 26] | 31 (56) [N = 55] | 0.05 | 1.00 |
| Body mass index, mean (SD), kg/m 2 | 30.6 (6.1) | 30.9 (7.7) | 0.03 | 0.82 |
SMD, standardized mean difference.
* Comparisons with SMD > 0.1 were suggestive of clinically relevant differences.
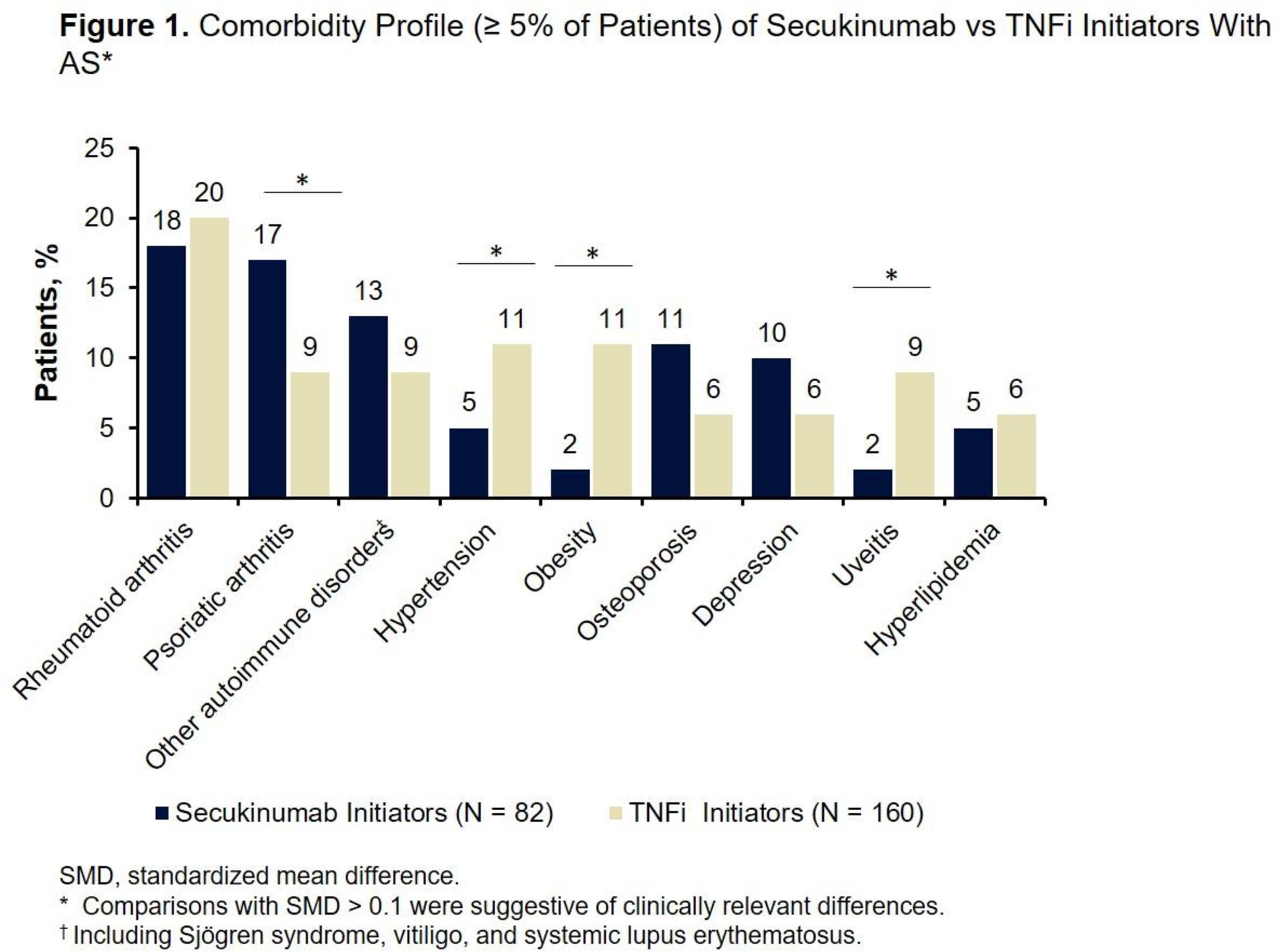
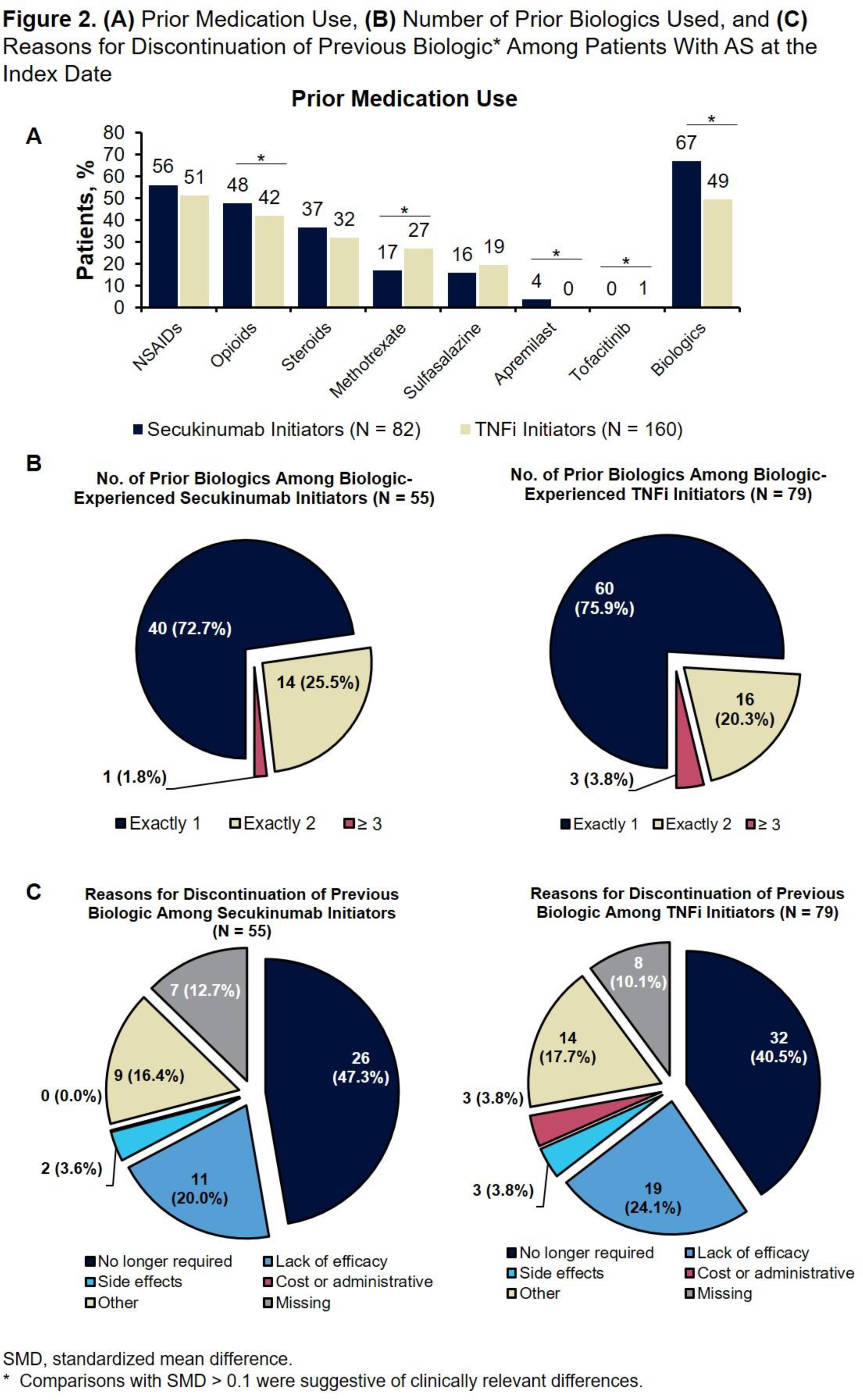
Conclusion: Secukinumab initiators with AS were younger and more opioid and biologic experienced, were more likely to have a PsA diagnosis, and were more likely to discontinue their previous biologic because the biologic was no longer required compared to patients who initiated TNFis.
Acknowledgments: This study was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ. Support for third-party writing assistance for this abstract, furnished by Kheng Bekdache, PhD, of Health Interactions, Inc, was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Disclosure of Interests: Howard Busch Speakers bureau: AbbVie, Amgen, Crescendo, Exagen, Genentech, Mallinckrodt, Novartis, Primus, Sanofi/Regeneron, and UCB, Jeffrey Curtis Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, UCB, Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, UCB, Peter Hur Employee of: Novartis Pharmaceuticals Corporation, Esther Yi Employee of: Novartis Pharmaceuticals Corporation