

Background: Secukinumab (SEC) is the first interleukin-17A inhibitor showing efficacy in both ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in randomised trials, but real-life data are lacking.
Objectives: In this prospective observational study, we evaluated the effectiveness and safety of SEC in patients with AS and PsA in a real-life setting.
Methods: From September 2018 to September 2019, data were collected from 168 consecutive outpatients at baseline (T0) and at 6 (T6) and 12 months (T12) after starting SEC (39 AS, 23%; 129 PsA, 77%).
Results: Significant improvement was seen at T6 and T12 for all clinical variables, including TJC, SJC, ESR, CRP, DAPSA, ASDAS-CRP, and BASDAI, as well as in patient-reported outcomes such as VAS-pain. By multivariable regression analysis, in AS patients high BASDAI at T0 correlated with diagnostic delay (R
2
=0.4; p=0.009) and peripheral joint involvement (R
2
=0.4; p=0.04). During follow-up, reduction of BASDAI positively correlated with high ESR (R
2
=0.65; p=0.04). ASDAS-CRP at T0 positively correlated with high ESR (R
2
=0.34; p=0.004). Reduction of ASDAS-CRP from T0 to T6 correlated with current smoking status (R
2
=0.42; p=0.0005). In PsA patients, reduction of DAPSA score from T0 to T12 negatively correlated with the presence of metabolic syndrome (R
2
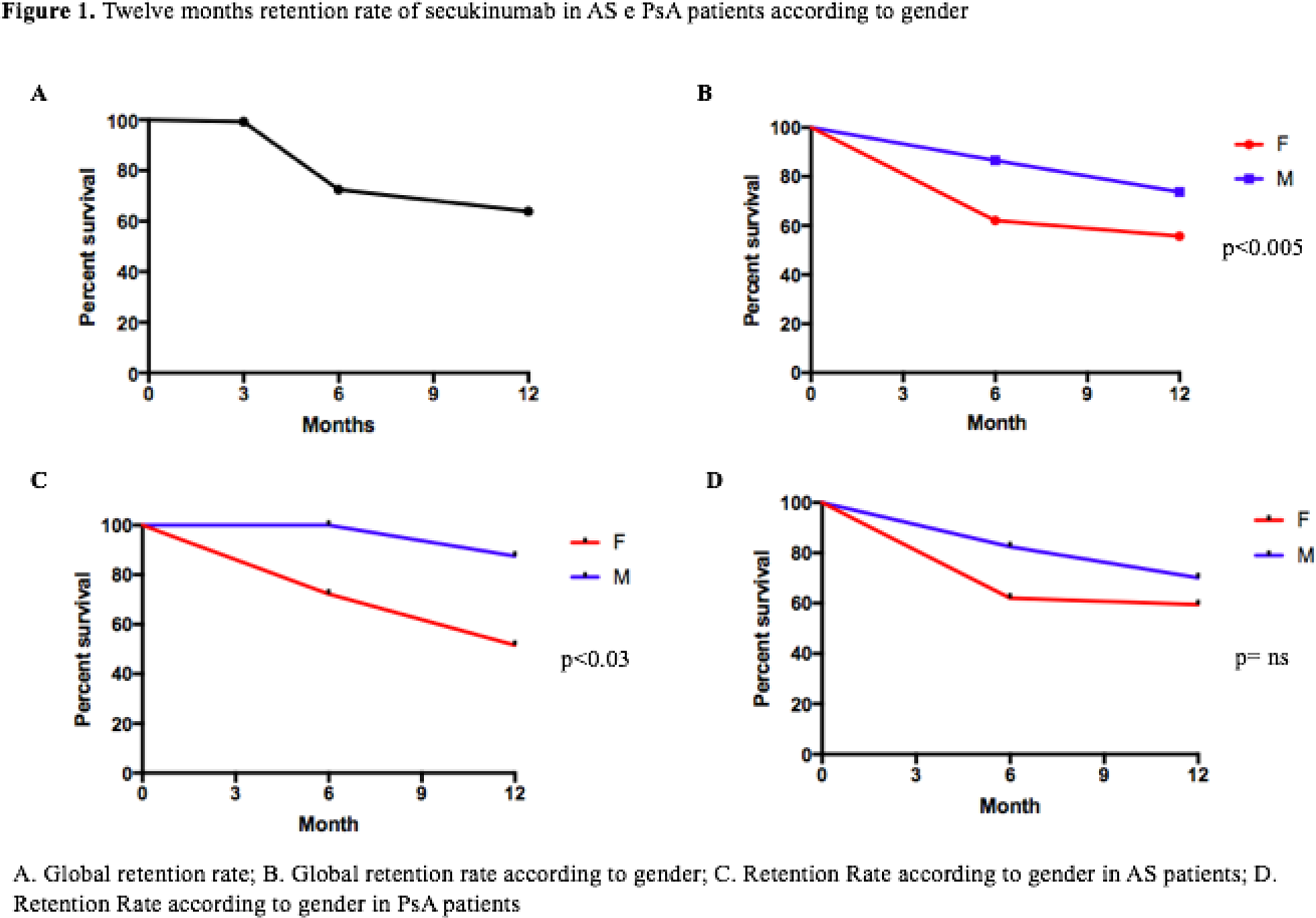
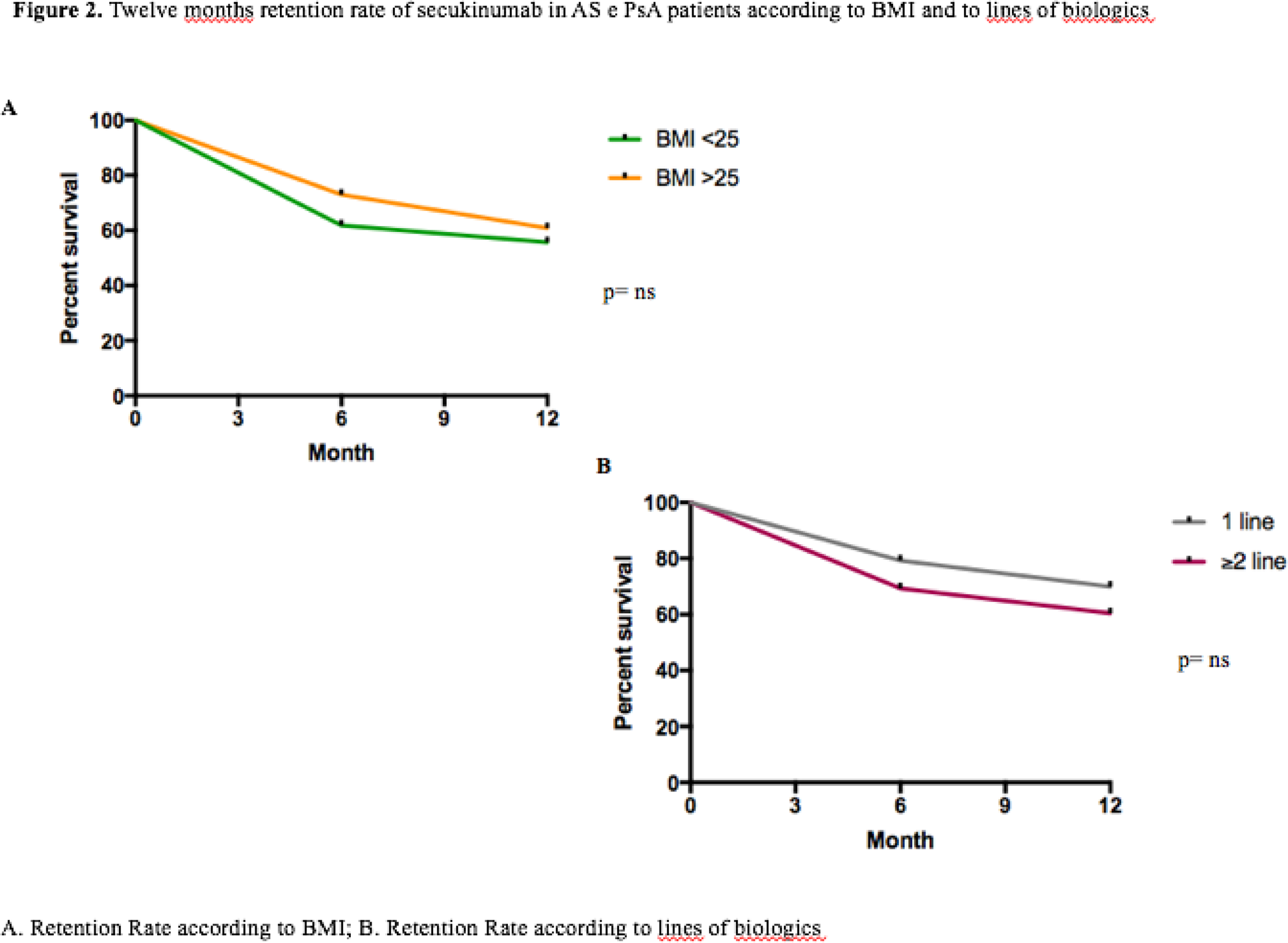
=0.41; p= 0.0025). Retention rate showed good drug survival and an influence of female sex (
Conclusion: We demonstrated the effectiveness and safety of SEC in patients with AS and PsA in a real-life setting for the first time. No gender differences were observed; however, less clinical improvement was seen in smokers and in patients with metabolic syndrome
REFERENCES:
No references.
Disclosure of Interests: Maria Sole Chimenti: None declared, giulia lavinia fonti: None declared, Paola Conigliaro: None declared, flavia sunzini: None declared, Rossana Scrivo: None declared, luca navarini: None declared, paola triggianese: None declared, giusy peluso: None declared, Palma Scolieri: None declared, rosalba caccavale: None declared, Andrea Picchianti-Diamanti: None declared, erica de martino: None declared, simonetta salemi: None declared, domenico birra: None declared, Alessio Altobelli: None declared, marino paroli: None declared, Vincenzo Bruzzese: None declared, Bruno Laganà: None declared, Elisa Gremese Speakers bureau: Abbvie, BMS, Celgene, Jannsen, Lilly, MSD, Novartis, Pfizer, Sandoz, UCB, fabrizio conti Speakers bureau: BMS, Lilly, Abbvie, Pfizer, Sanofi, Antonella Afeltra: None declared, Roberto Perricone: None declared