

Background: Golimumab ( GLM ) is the latest anti-TNFα to be indicated for treating rheumatoid arthritis ( RA ), psoriatic arthritis ( PsA ) and axial spondyloarthritis ( axSpA ). The GO-PRACTICE study was performed in France at the request of the French Health Authorities, for the reevaluation of GLM in real-life.
Objectives: The primary objective was to estimate GLM persistence at 2 years from initial prescription. This abstract focuses on a post-hoc analysis of the factors linked to GLM discontinuation in axSpA patients.
Methods: Observational, prospective, multicenter study, that consecutively recruited adult patients with RA, PsA and axSpA who were newly prescribed GLM. Patients were followed-up for 2 years and outcomes data were collected at baseline ( BL ), 1 and 2 years. Patients’ sociodemographic characteristics, disease history, comorbidities and treatment history were also collected at BL. Persistence was estimated with the Kaplan-Meier method. Cox proportional hazard models were used to assess factors associated with persistence. Selected BL characteristics were studied in univariate models, where those associated with p -value <0.20 were included in multivariate analysis. Significance level was set at p <0.05.
Results: 478 patients with axSpA were included from Jan 2015 to Mar 2016. Mean age was 43 years and 55% were female; 61% of patients were biologic-naïve (BN, n=291) and 39% (n=187) were biologic-pretreated (BP). Median time-elapsed in years since axSpA diagnosis was 1.7 (range 0–45.1) and 6.9 (range 0.2–51.8) in BN and BP patients, respectively ( P <0.001); 97% patients were prescribed 50 mg GLM monthly and co-treatments included DMARD (34%), corticosteroids (17%) and NSAIDs/analgesics (90%).
Cumulative persistence probability of GLM at 2-years was 52.6% (
Logistic model results for variables of interest and their link to GLM discontinuation in axSpA
| Factor | Modalities | χ 2 (p) | Hazard ratio (HR) | 95% CI |
|---|---|---|---|---|
| HR following univariate analysis (p>0.20) | ||||
| Age | Continuous variable | 0.520 | 1.00 | 0.99–1.02 |
| Disease duration | 0.401 | 1.01 | 0.99–1.03 | |
| Inflammatory bowel disease | Yes vs. No | 0.277 | 0.74 | 0.43–1.28 |
| Gastrointestinal disease | 0.344 | 1.27 | 0.78–2.06 | |
| Uveitis | 0.237 | 0.80 | 0.55–1.16 | |
| Psoriasis | 0.238 | 0.92 | 0.64–1.31 | |
| HR following multivariate analysis (variables with p<0.20 at univariate analysis) | ||||
| Gender | Female vs. Male | < 0.001 | 1.92 | 1.43–2.56 |
| Biologics history | Pretreated vs. naïve | 0.007 | 1.45 | 1.11–1.90 |
| Serum CRP | Continuous variable | 0.177 | 0.99 | 0.98–1.00 |
| DMARD history | Yes vs. No | 0.062 | 1.37 | 0.99–1.90 |
| Ongoing corticosteroids | 0.693 | 1.08 | 0.73–1.61 | |
| Anemia | 0.170 | 1.82 | 0.78–4.24 | |
| Kidney Disease | 0.508 | 1.50 | 0.45–4.97 | |
| Other physical illness | 0.435 | 1.28 | 0.69–2.34 | |
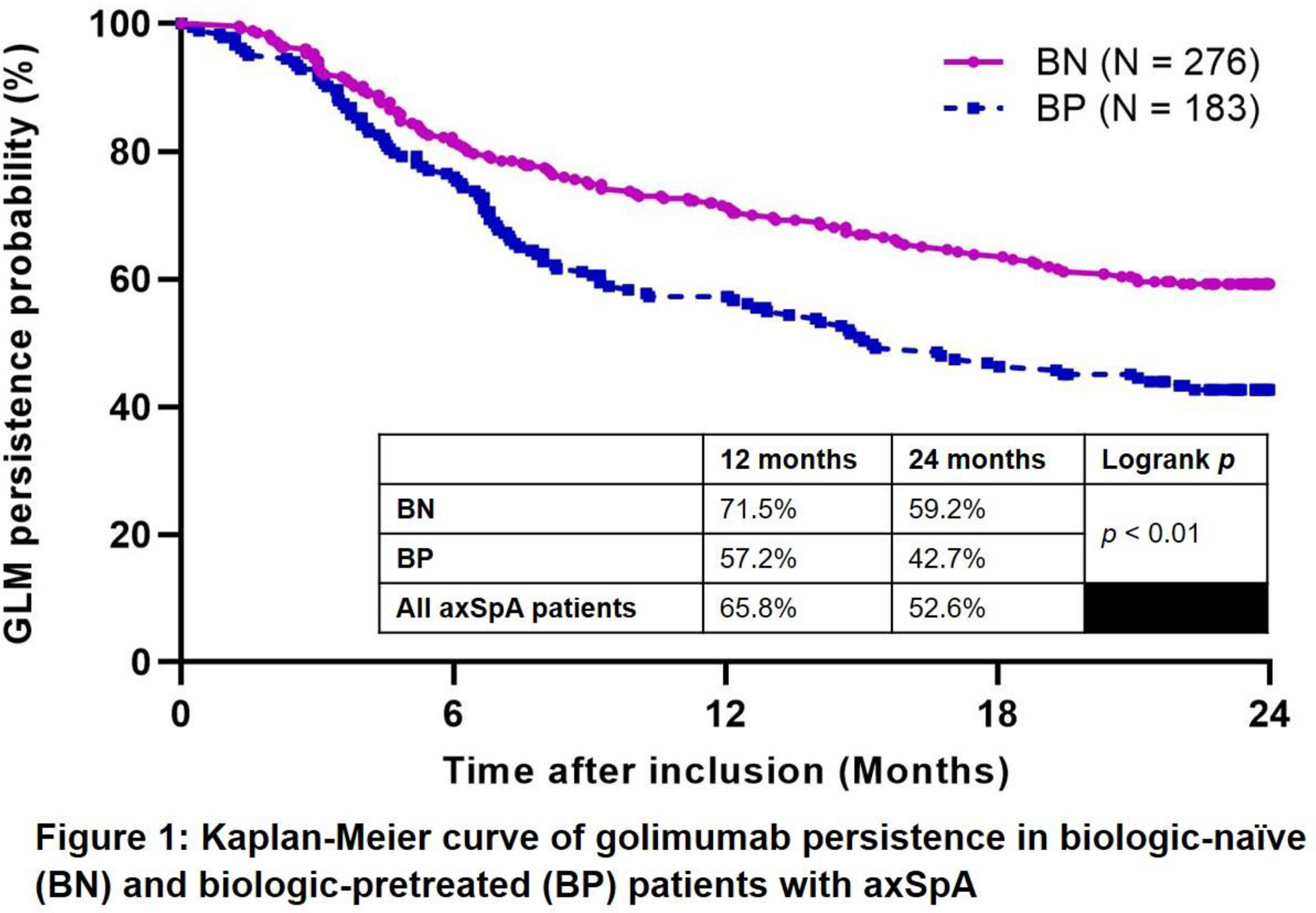
Conclusion: 2-year GLM persistence in axSpA patients was 52.6%. Females and those who were biologics-pretreated were at greater risk for discontinuing GLM before 2 years.
Disclosure of Interests: Philippe Bertin Consultant of: MSD France, Philippe Goupille Grant/research support from: AbbVie, Amgen, Biogen, BMS, Celgene, Chugai, Lilly, Janssen, Medac, MSD France, Nordic Pharma, Novartis, Pfizer, Sanofi and UCB, Consultant of: AbbVie, Amgen, Biogen, BMS, Celgene, Chugai, Lilly, Janssen, Medac, MSD France, Nordic Pharma, Novartis, Pfizer, Sanofi and UCB, Speakers bureau: AbbVie, Amgen, Biogen, BMS, Celgene, Chugai, Lilly, Janssen, Medac, MSD France, Nordic Pharma, Novartis, Pfizer, Sanofi and UCB, Florence Tubach Grant/research support from: Florence TUBACH is head of the Centre de Pharmacoépidémiologie (Cephepi) of the Assistance Publique – Hôpitaux de Paris and of the Clinical Research Unit of Pitié-Salpêtrière hospital, both these structures have received research funding, grants and fees for consultant activities from a large number of pharmaceutical companies, that have contributed indiscriminately to the salaries of its employees. Florence Tubach didn’t receive any personal remuneration from these companies., Eric Lespessailles Consultant of: Amgen, Celgene, Lilly, MSD France, Novartis, UCB, Speakers bureau: Amgen, Celgene, Lilly, MSD France, Novartis, UCB, Naoual HARID Employee of: MSD France, Saannya Sequeira Consultant of: MSD France, Jean-Marie Fayette Consultant of: MSD France, Bruno Fautrel Grant/research support from: AbbVie, Lilly, MSD, Pfizer, Consultant of: AbbVie, Biogen, BMS, Boehringer Ingelheim, Celgene, Lilly, Janssen, Medac MSD France, Nordic Pharma, Novartis, Pfizer, Roche, Sanofi Aventis, SOBI and UCB, René-Marc Flipo Consultant of: Johnson and Johnson, MSD France, Novartis, Sanofi, Speakers bureau: Johnson and Johnson, MSD France, Novartis, Sanofi