

Background: For inflammatory arthritis, drug retention is accepted as an important indicator of the effectiveness and safety of biological drugs.
Objectives: The objective of this study was to determine of the effects first and overall bDMARD drug retention rate during concomitant csDMARD in psoriatic arthritis (PsA).
Methods: HUR-BIO (Hacettepe University Rheumatology Biologic Registry) is a prospective, single center database of biological treatments since 2005. All PsA patients (469) who enrolled in HUR-BIO registry and prescribed at least once biologic DMARD (bDMARD) were included in the study. The subjects were divided into two groups depending on whether or not to use csDMARDs (methotrexate, sulphasalazine or leflunomide) at the last control visit. Demographic, clinical and therapeutic data were collected from this database. Baseline disease activity before the first bDMARD initiation was assessed with DAPSA and PsAID-12.
Demographics, baseline and follow-up clinical characteristics of PsA patients
| With concomitant csDMARD n=288 | Without concomitant csDMARD n=167 | p | |
|---|---|---|---|
| Age (mean, SD) | 48.9 (12.1) | 45.1 (12.4) | 0.002 |
| Female n (%) | 204 (70.8) | 113 (67.7) | 0.48 |
| PsA disease duration (med, IQR) | 7 (13) | 7 (14.5) | 0.53 |
| Age at PsA diagnosis (mean, SD) | 39 (11.7) | 40.8 (12.9) | 0.81 |
| BMI (mean, SD) | 29.6 (6.0) | 29.2 (5.9) | 0.53 |
| Axial involvement n (%) | 68 (34.0) | 37 (44.6) | 0.09 |
| HLA-B 27 (+) n (%) | 21/76 (27.6) | 17/69 (24.6) | 0.68 |
| Duration of use first bDMARD (months) (med, IQR) | 22.4 (51.3) | 14.1 (31.4) | 0.003 |
| Duration of use overall bDMARD (months) (med, IQR) | 56.8 (69.4) | 24.4 (57.7) | <0.001 |
| Switching bDMARD (+) n (%) | 148 (52.5) | 71 (42.8) | 0.04 |
| Initial DAPSA 1 (med, IQR) | 19.4 (11.7) | 17.3 (10.2) | 0.04 |
| Initial PSAID 2 (med, IQR) | 5.7 (3) | 5.6 (2.8) | 0.55 |
| Final visit PSAID 3 (med, IQR) | 2.8 (3.8) | 2 (5.7) | 0.41 |
| Final visit DAPSA
4
(med, IQR)
| 10.2 (12.4) | 12.5 (12.5) | 0.01 |
| Remission (n,%) | 61 (23.9)
| 27 (17.6)
| 0.13 |
| Low disease activity | 111 (43.5) | 59 (38.6) | |
| Moderate disease activity | 72 (28.2) | 58 (37.9) | |
| High disease activity | 11 (4.3) | 9 (5.9) |
1 : n=249, 2 : n=214, 3 : n=109, 4 : n=408
p<0.001
p=0.003
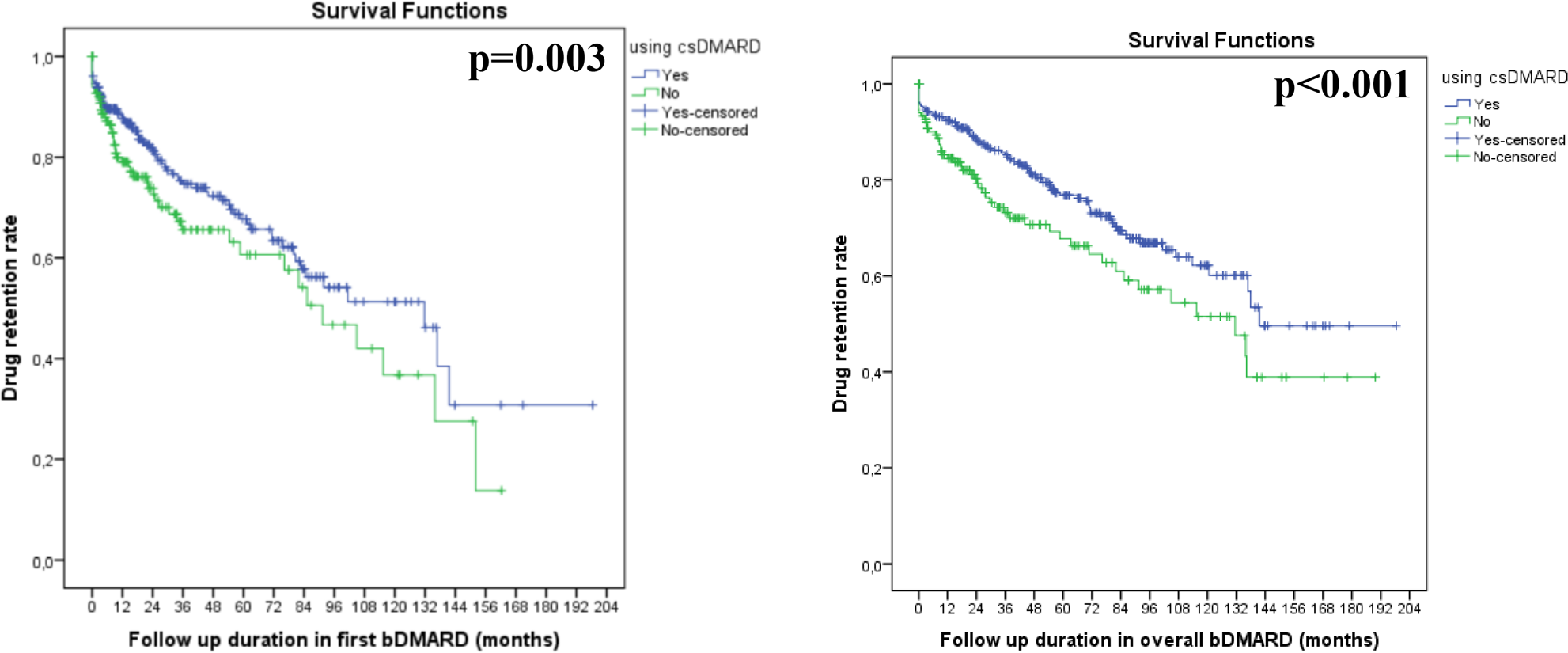
Results: HUR-BIO PsA registry included 469 PsA patients. Baseline, clinical characteristics and follow-up parameters were given in Table. The using overall bDMARD were adalimumab 294 (62.0%), etanercept 135 (28.8%), infliximab 119 (25.4%), certolizumab pegol 107 (22.8%), secukinumab 67 (14.3%), golimumab 58 (12.4%), ustekinumab 25 (5.3%) and tofacitinib 11 (2.3%). Two hundred eighty eight (61.4%) patients used concomitant cDMARDs [methotrexate 176 (37.5%), leflunomide 94 (20.0%), sulphasalazine 35 (7.5%) and two csDMARD combination 17 (3.6%)]. The first-year retention rate of first bDMARD with or without concomitant csDMARDs were 88% and 80%, respectively. The median duration of first bDMARD retention with or without concomitant csDMARDs were 131.7 and 91.4 months, respectively (Figure). The first-year retention rate of overall bDMARDs with or without concomitant csDMARDs were 92% and 85%, respectively. The median drug retention rate of overall bDMARD in using csDMARD and not using csDMARD were 141.5 and 131.5 months, respectively. Retention rates (both for first bDMARD and overall bDMARDs) were significantly higher in concomitant csDMARDs using group (p=0.003 for first bDMARD retention, p<0.001 for overall bDMARDs retention, log-rank; Figure). In concomitant csDMARD using group, no differences were identified methotrexate or other csDMARDs.
Conclusion: In this study, csDMARDs, either methotrexate or leflunomide/sulphasalazine have additional effect for both retention rate and treatment response of bDMARDs. On the other hand, using bDMARD monotherapy is relatively higher than rheumatoid artritis (1).
Drug retention rate of the first bDMARD and overall bDMARDs according to concomitant csDMARD use ( csDMARD:conventional synthetic Disease Modifiying Antirheumatic Drug )
Disclosure of Interests: Emine Duran: None declared, Emre Bilgin: None declared, Ertugrul Cagri Bolek: None declared, Gözde Kübra Yardimci: None declared, Bayram Farisoğullari: None declared, Levent Kiliç: None declared, Ali Akdoğan: None declared, Omer Karadag: None declared, Şule Arpaş Bilgen: None declared, Sedat Kiraz: None declared, Ali İhsan Ertenli: None declared, Umut Kalyoncu Consultant of: Abbvie, Amgen, Janssen, Lilly, Novartis, UCB