

Background: Reactive oxygen species (ROS) are one of the significant factors of chemical or physical cell signaling in a wide variety of cell types including skeletal cells. Receptor activator of NF-βB ligand (RANKL) induces generation of intracellular ROS, which act as second messengers in RANKL-mediated osteoclastogenesis. Dual oxidase maturation factor 1 (Duoxa1) was first identified as a Drosophila Numb-interacting protein (NIP), and has been associated with the maturation of ROS generating enzymes including dual oxidases (Duox1 and Duox2). In the progression of osteoclast differentiation using mouse bone marrow-derived macrophages (BMMs), we identified that only Duoxa1 level showed an effective change upon RANKL stimulation, but not Duox1, Duox2, and Duoxa2.
Objectives: we hypothesized that Duoxa1 could independently act as a second messenger for RANKL stimulation and regulate ROS production during osteoclast differentiation.
Methods: Using siRNA or retrovirus transduction and knockdown of Duoxa1 via siRNA
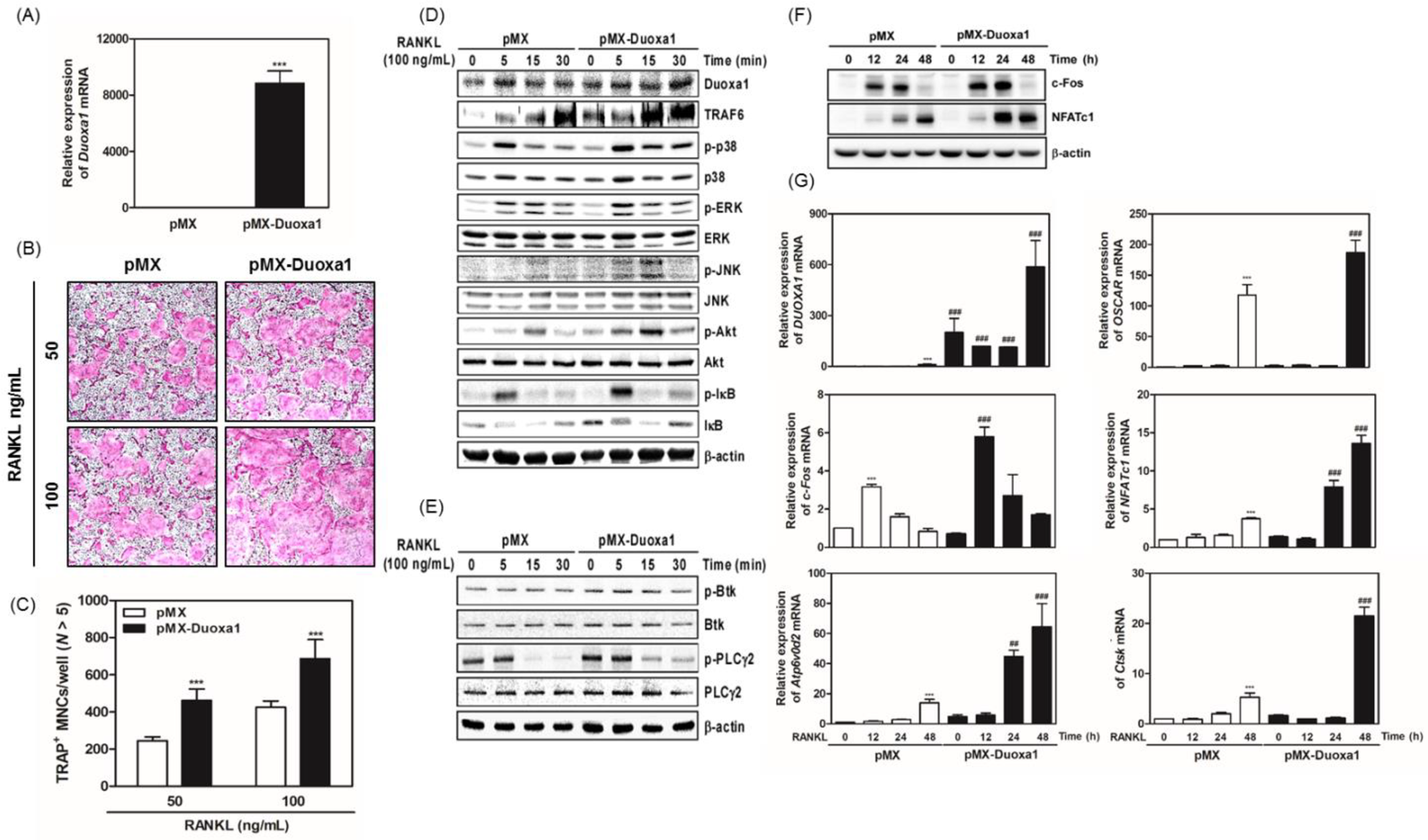
Results: Duoxa1 level gradually increased during RANKL-induced osteoclast differentiation. We found that Duoxa1 regulated RANKL-stimulated osteoclast formation and bone resorption positively. knockdown of Duoxa1 via siRNA decreased the RANKL-induced ROS production. During Duoxa1-related control of osteoclastogenesis, activation of tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6)-mediated early signaling molecules including MAPKs, Akt, IβB, Btk, and PLC 2 was affected, which sequentially modified the mRNA or protein expression levels of key transcription factors in osteoclastogenesis, such as c-Fos and NFATc1, as well as mRNA expression of osteoclast-specific markers including OSCAR, ATP6v0d2, and CtsK.
Conclusion: Overall, our data indicate that Duoxa1 plays a crucial role in osteoclastogenesis via regulating RANKL-induced intracellular ROS production and activating TRAF6-mediated signaling.
Disclosure of Interests: None declared