

Background: Erdheim-Chester disease (ECD) is a rare non-Langerhans histiocytosis. Combined treatment with anakinra (ANK) and targeted MAPK-inhibiting therapies (vemurafenib – VMF - or cobimetinib - CBM) has been recently used to treat severe cases of ECD.
Objectives: To evaluate the safety and the clinical and radiological efficacy of ANK, targeted and combined treatments in ECD patients in a real-world setting.
Methods: ECD patients followed at our Center who received at least one targeted therapy and/or ANK were selected. Data about disease characteristics, adverse reactions, clinical and radiological efficacy (by means of CT, CT/PET, MRI when appropriate and repeated 6 months apart) were collected.
Results: Among the 48 ECD patients followed up at our Center, 27 were treated with at least one drug between VMF and CBM, accounting for a total of 22 and 10 therapy courses respectively. 12 patients were treated with ANK as monotherapy or combination treatment (a total of 17 therapy courses). Disease characteristics and adverse reactions to the treatments are shown in
Disease characteristics and therapy-related adverse reactions of Erdheim-Chester patients treated with vemurafenib, cobimetinib and/or anakinra.
| Vemurafenib (n=19 ) | Cobimetinib (n=10 ) | Anakinra (n=12 ) | |
|---|---|---|---|
| Clinical Manifestations | |||
| Cardiovascular | 79% | 70% | 75% |
| Retroperitoneal | 84% | 60% | 42% |
| Pleuropulmonary | 63% | 80% | 42% |
| Neurological and/or orbital | 90% | 90% | 92% |
| Adverse reactions | 74% | 60% | 25% |
| Renal | 26% | 10% | 0% |
| Cutaneous | 26% | 30% | 17% |
| Systemic Inflammation | 11% | 10% | 0% |
| Cardiovascular | 11% | 10% | 0% |
| Gastrointestinal | 0% | 30% | 0% |
| Haematological | 0% | 10% | 0% |
| Herpes Zoster | 0% | 0% | 8% |
Efficacy and discontinuation rates of monotherapy and combined treatment in Erdheim-Chester disease. * Referred to imaging re-staging.
| Therapy courses | Clinical efficacy | Improving disease * | Stable disease * | Progressive disease * | Discontinuation due to toxicity | Discontinuation due to inefficacy | |
|---|---|---|---|---|---|---|---|
| Vemurafenib | 19 | 93% | 73% | 27% | 0% | 37% | 0% |
| Cobimetinib | 6 | 80% | 100% | 0% | 0% | 50% | 0% |
| Anakinra | 10 | 86% | 0% | 25% | 75% | 20% | 40% |
| Vemurafenib + Anakinra | 3 | 100% | 100% | 0% | 0% | 0% | 0% |
| Cobimetinib + Anakinra | 4 | 100% | 100% | 0% | 0% | 25% | 0% |
Mean acute phase reactant levels and concomitant prednisone dose in Erdheim-Chester disease patients treated with vemurafenib before and after the initiation of anakinra.
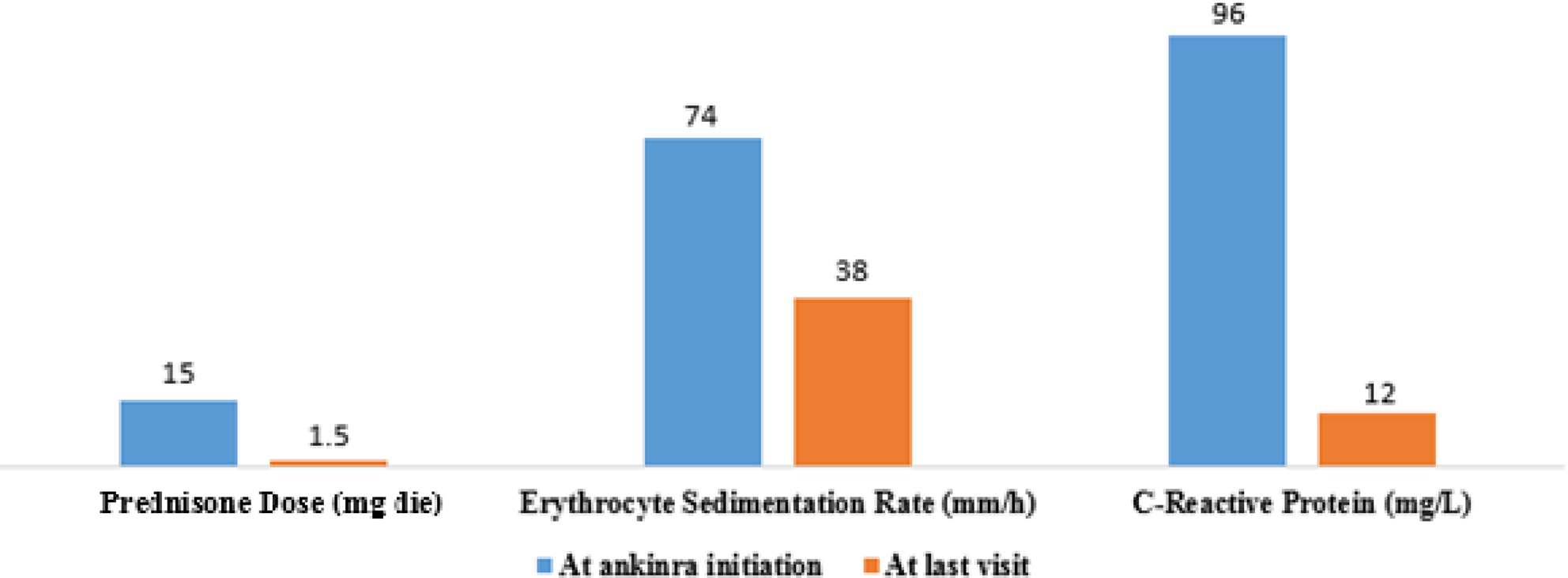
Conclusion: Combined treatment had a higher clinical and radiological efficacy than ANK monotherapy and led to a reduction in targeted therapies-related toxicities, acute phase reactant levels and concomitant prednisone dose.
REFERENCES:
[1]Efficacy and improved tolerability of combination therapy with interleukin-1 blockade and MAPK pathway inhibitors for the treatment of Erdheim-Chester disease; Campochiaro et al; Ann Rheum Dis. 2019; 10.1136/annrheumdis-2019-216610
Disclosure of Interests: Nicola Farina: None declared, Corrado Campochiaro Speakers bureau: Novartis, Pfizer, Roche, GSK, SOBI, Alessandro Tomelleri: None declared, Giacomo De Luca Speakers bureau: SOBI, Novartis, Celgene, Pfizer, MSD, Giulio Cavalli Speakers bureau: SOBI, Novartis, Pfizer, Lorenzo Dagna Grant/research support from: Abbvie, BMS, Celgene, Janssen, MSD, Mundipharma Pharmaceuticals, Novartis, Pfizer, Roche, SG, SOBI, Consultant of: Abbvie, Amgen, Biogen, BMS, Celltrion, Novartis, Pfizer, Roche, SG, and SOBI