

Background: Patient persistence with biologic treatment of rheumatoid arthritis (RA), psoriatic arthritis (PsA) and ankylosing spondylitis (AS) (collectively inflammatory arthritis, IA) may be considered a proxy for efficacy, safety and treatment satisfaction. Patients who discontinue their first line of subcutaneous tumor necrosis factor alpha inhibitors (SC-TNFis) and switch to at least one subsequent line of SC-TNFi can be defined as cyclers.
Objectives: To assess persistence by line of therapy in Swedish IA patients cycling on SC-TNFis.
Methods: Using data from the Swedish Health Data Registers, adult IA patients initiating treatment with any available SC-TNFi (adalimumab, etanercept, certolizumab, or golimumab) between May 1 st 2010 and October 31 st 2016 were eligible for inclusion. Treatment persistence was derived using information from filled prescriptions (e.g. dispensing date, pack information, and defined daily dose) with a 60-day grace period. Analyses were restricted the first two lines of treatments (i.e. 1 st and 2 nd ) in patients defined as SC-TNFi cyclers. Persistence estimates across treatment lines were assessed graphically using Kaplan-Meier curves. Unadjusted and adjusted marginal Cox proportional hazards models were fitted to estimate the relative risk of discontinuation across treatment lines, using robust sandwich covariance matrix estimates to account for intrapatient dependence (i.e. multiple treatment lines per patient). Covariates in the adjusted analysis included age, gender, diagnosis, year of treatment initiation, comorbidities, co-medication, and the number of specialized outpatient care visits and inpatient stays.
Results: Of 11,668 patients initiating SC-TNFi treatment, 3,181 patients were identified as cyclers. Among these, a majority were female (68%) with a mean age of 50 years; 46%, 28%, and 26% were diagnosed with RA, AS and PsA, respectively.
Relative risk of discontinuing subcutaneous Tumor Necrosis Factor-α inhibitor treatment for IA in 2nd line treatment compared to 1st line treatment, by analysis population
| Analysis population | N | Unadjusted analysis, HR [95%CI] | Adjusted analysis, HR [95%CI] |
|---|---|---|---|
| Cyclers overall | 3,181 | 0.60 [0.57, 0.63] | 0.59 [0.56, 0.62] |
| RA | 1,479 | 0.62 [0.57, 0.67] | 0.61 [0.56, 0.66] |
| PsA | 891 | 0.60 [0.54, 0.67] | 0.59 [0.53, 0.65] |
| AS | 811 | 0.58 [0.52, 0.64] | 0.55 [0.50, 0.61] |
HR: Hazard Ratio, 95%CI: 95% confidence interval
Persistence, among cyclers, with subcutaneous Tumor Necrosis Factor-α inhibitors treatment for IA by line of treatment
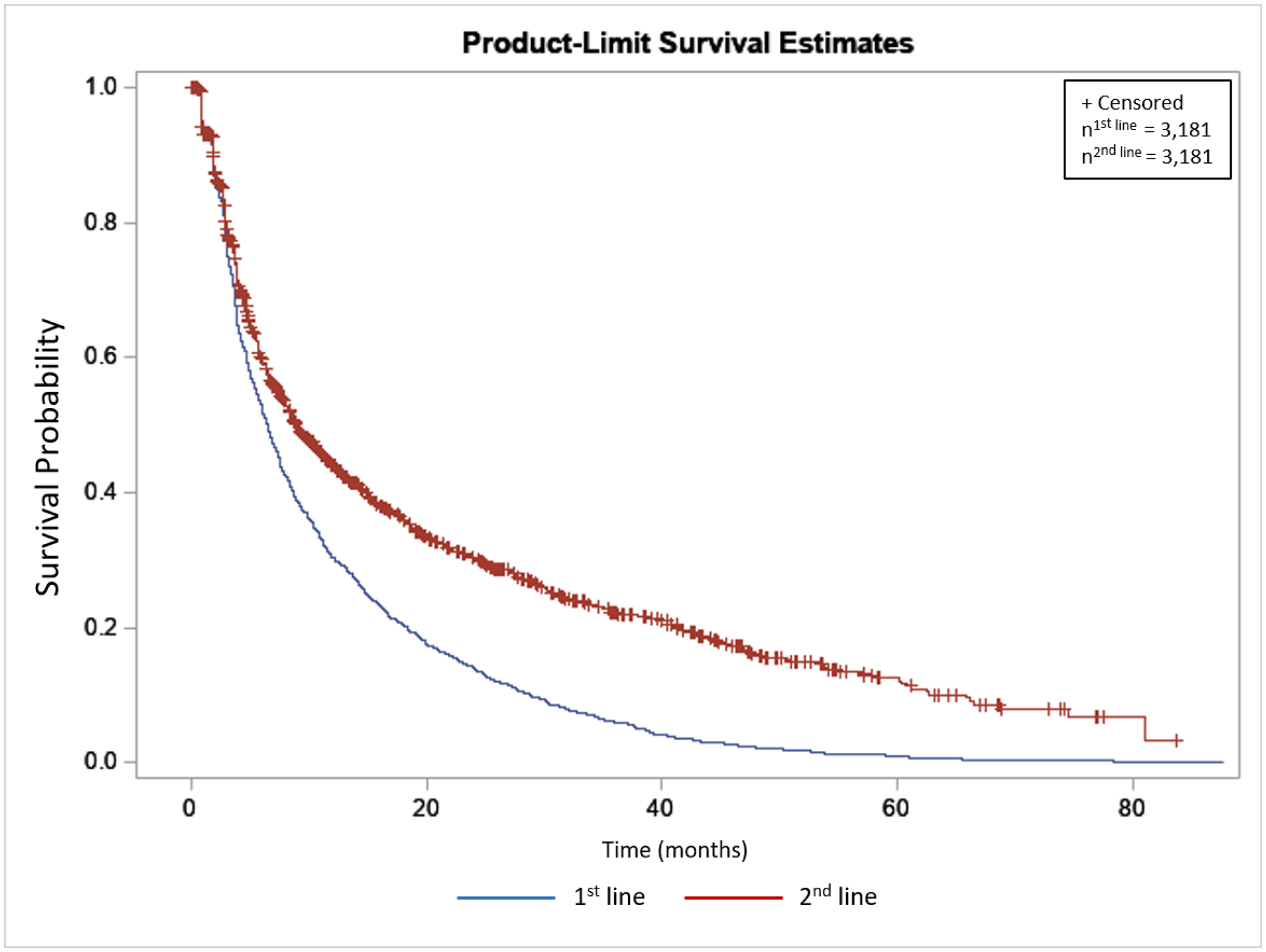
Conclusion: In this preliminary analysis of IA patients cycling on SC-TNFis, persistence was greater in 2 nd line compared to 1 st line treatment. The finding was consistent across all IA indications. Hence, IA patients who fail to respond, lose response, or for other reasons discontinue their 1 st line treatment may still benefit from switching to an alternative SC-TNFi as a 2 nd line therapy.
Disclosure of Interests: Johan Dalén Consultant of: Merck & Co., Inc. in conjunction with the development of this abstract. JD is an employee of ICON plc. ICON plc have received funding from several pharmaceutical companies involved in the marketing products for treatment of inflammatory arthritis., Amy Puenpatom Shareholder of: shareholder at Merck & Co, Inc, Employee of: Employed at Merck & Co, Inc., Karin Luttropp Consultant of: Merck & Co., Inc. in conjunction with the development of this abstract. KL is an employee of ICON plc. ICON plc have received funding from several pharmaceutical companies involved in the marketing products for treatment of inflammatory arthritis., Christopher Black Shareholder of: I own shares of MSD, Employee of: I am an employee of MSD