

Background: Adherence is a serious problem in treatment of inflammatory diseases. To measure adherence, caps that record medication bottle openings may be superior to capsule counts (1). In the ongoing two-year GLORIA trial on the addition of low-dose (5 mg) prednisolone or placebo to standard of care in elderly patients (65+ years) with rheumatoid arthritis, adherence was measured in both ways during the whole trial.
Objectives: To describe adherence patterns, and to compare adherence as assessed with adherence caps and with capsule counts in the GLORIA trial.
Methods: The recorded adherence patterns of patients (blinded for treatment group) were classified according to descriptive categories. Overall adherence according to number of bottle openings was compared with adherence according to the capsule count. Good adherence was defined as 80%: i.e. for caps 80% of days one opening recorded, and for counts less than 20% of prescribed tablets returned at the subsequent visit. Each patient has a maximum of 8 periods of 90 days.
Results: Trial inclusion has closed in 2018 at 452 patients; the current dataset contains adherence data of 385 patients. Mean number of recorded 90-day periods per patient was 4 (range 1-8). Based on capsule counts over all periods, 90% of the patients met the 80% threshold of adherence; based on cap data only 31% met this criterion.
The four adherence patterns are shown in a calendar matrix, with yellow for zero, green for one and blue for ≥two openings on a day (
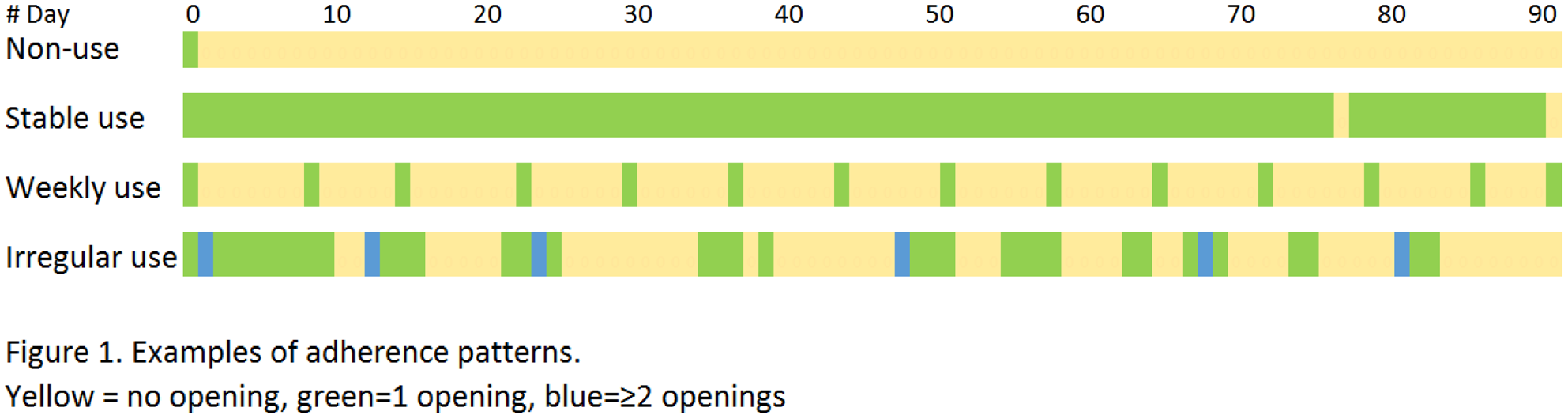
Patients were categorized according to the opening pattern seen in at least 50% of assessed periods:
| 32% non-use | (<20% of the days an opening); |
| 26% stable use | (≥80% of the days 1 opening); |
| 40% irregular use | (different adherence patterns, in or between periods); |
| 2% weekly use | (1 opening per week). |
Conclusion: In our trial of elderly rheumatoid arthritis patients, patients appeared to be mostly adherent according to conventional capsule counts. Results from adherence caps were highly discrepant with the capsule counts, with patterns suggesting patients did not use the bottle for daily dispensing, despite specific advice to do so.
REFERENCES:
[1] El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol 2016;82:268-79.
Acknowledgments: The GLORIA project is funded by the European Union’s Horizon 2020 research and innovation programme under the topic ‘’Personalizing Health and Care’’, grant agreement No 634886.
Disclosure of Interests: Linda Hartman: None declared, Sabrina Paolino: None declared, Reinhard Bos: None declared, Daniela Opris-Belinski Speakers bureau: as declared, Marc R Kok Grant/research support from: BMS and Novartis, Consultant of: Novartis and Galapagos, Hanneke Griep-Wentink: None declared, Ruth Klaasen: None declared, Cornelia Allaart: None declared, George Bruyn: None declared, Hennie Raterman Grant/research support from: UCB, Consultant of: Abbvie, Amgen, Bristol-Myers Sqibb, Cellgene and Sanofi Genzyme, Marieke Voshaar Grant/research support from: part of phd research, Speakers bureau: conducting a workshop (Pfizer), Nuno Gomes: None declared, Rui Pinto: None declared, Thomas Klausch: None declared, WIllem Lems Grant/research support from: Pfizer, Consultant of: Lilly, Pfizer, Maarten Boers: None declared