

Background: EULAR recommendation announced that biological disease-modifying antirheumatic drugs (bDMARDs) and janus kinase inhibitors (JAKi) are considered as equivalent in the treatment of rheumatoid arthritis (RA). However, we still lack reliable evidence of direct comparison between these agents’ retention, which may reflect both effectiveness and safety.
Objectives: The aim of this multi-center (7 university-related hospitals), retrospective study is to clarify retention rates and reasons for discontinuation of 7 bDMARDs and tofacitinib (TOF), one of the JAKi, in both bDMARDs-naïve and bDMARDs-switched cases.
Methods: This study assessed 3,897 patients and 4,415 treatment courses of with bDMARDs and TOF from 2001 to 2019 (2,737 bDMARDs-naïve patients and 1,678 bDMARDs-switched patients [59.5% switched to their second agent], female 82.3%, baseline age 57.4 years, disease duration 8.5 years; rheumatoid factor positivity 78.4%; DAS28-ESR 4.3; concomitant prednisolone [PSL] 6.1 mg/day [42.4%] and methotrexate [MTX] 8.5 mg/week [60.9%]). Treatment courses included abatacept (ABT; n=663), adalimumab (ADA; n=536), certolizumab pegol (CZP; n=226), etanercept (ETN; n=856), golimumab (GLM; n=458), infliximab (IFX; n=724), tocilizumab (TCZ; n=851), and TOF (n=101/only bDMARDs-switched cases). Reasons for discontinuation were classified into four categories by each attending physician: 1) lack of effectiveness, 2) toxic adverse events, 3) non-toxic reasons, and 4) remission. Retention rates of each discontinuation reason were estimated at 36 months using the Kaplan-Meier method and adjusted for potential clinical confounders (age, sex, disease duration, concomitant PSL and MTX, starting date and number of switched bDMARDs) using Cox proportional hazards modeling.
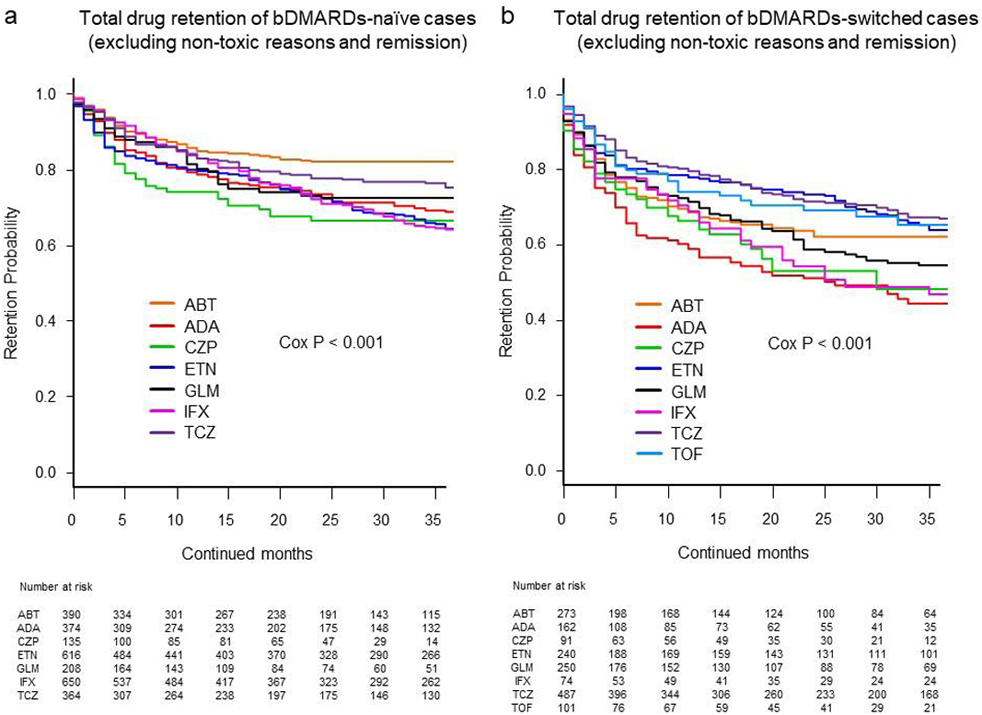
Results: Adjusted drug retention rates for each discontinuation reason were as follows: lack of effectiveness in the bDMARDs-naïve group (from 70.8% [CZP] to 85.1% [ABT]; P=0.001 between agents) and the bDMARDs-switched group (from 52.8% [CZP] to 78.7% [TCZ]; P<0.001 between agents). Toxic adverse events in the bDMARDs-naïve group (from 86.9% [IFX] to 96.3% [ABT]; P<0.001 between agents) and the bDMARDs-switched group (from 81.1% [ADA] to 95.4% [ETN]; P=0.01 between agents). Finally, overall retention rates excluding discontinuation for non-toxic reasons or remission ranged from 64.2% (IFX) to 82.0% (ABT) (P<0.001 between agents) in the bDMARDs-naïve group (figure a) and from 44.2% (ADA) to 66.8% (TCZ) (P<0.001 between agents) in the bDMARDs-switched group (figure b).
Conclusion: Remarkable differences were observed in drug retention of 7 bDMARDs and TOF between bDMARDs-naïve and bDMARDs-switched cases.
Disclosure of Interests: Kosuke Ebina Grant/research support from: KE has received research grants from Abbie, Asahi-Kasei, Astellas, Chugai, Eisai, Ono Pharmaceutical, and UCB Japan., Employee of: KE is affiliated with the Department of Musculoskeletal Regenerative Medicine, Osaka University, Graduate School of Medicine, which is supported by Taisho., Speakers bureau: KE has received payments for lectures from Abbie, Asahi-Kasei, Astellas, Ayumi, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Janssen, Mitsubishi-Tanabe, Ono Pharmaceutical, Sanofi, and UCB Japan., Toru Hirano Grant/research support from: TH received a research grant and/or speaker fee from Astellas, Chugai, Nippon Shinyaku, Abbvie, Eisai, and Ono Pharmaceutical, Speakers bureau: TH received a research grant and/or speaker fee from Astellas, Chugai, Nippon Shinyaku, Abbvie, Eisai, and Ono Pharmaceutical, Yuichi Maeda Grant/research support from: YM received a research grant and/or speaker fee from Eli Lilly, Chugai, Pfizer, Bristol-Myers Squibb, and Mitsubishi-Tanabe, Speakers bureau: YM received a research grant and/or speaker fee from Eli Lilly, Chugai, Pfizer, Bristol-Myers Squibb, and Mitsubishi-Tanabe, Wataru Yamamoto: None declared, Motomu Hashimoto Grant/research support from: Bristol-Myers Squibb, Eisai, and Eli Lilly and Company., Speakers bureau: Bristol-Myers Squibb and Mitsubishi Tanabe Pharma., Koichi Murata Grant/research support from: KMurata belong to a department that has been financially supported by four pharmaceutical companies (Mitsubishi-Tanabe, Chugai, AYUMI and UCB Japan)., Employee of: KMurata belong to a department that has been financially supported by four pharmaceutical companies (Mitsubishi-Tanabe, Chugai, AYUMI and UCB Japan)., Speakers bureau: KMurak has received speaking fees, and/or consulting fees from Eisai Co. Ltd, Chugai Pharmaceutical Co. Ltd., Pfizer Japan Inc, Bristol-Myers Squibb, Mitsubishi-Tanabe Pharma Corporation, UCB, Daiichi Sankyo Co. Ltd. and Astellas Pharma Inc., Tohru Takeuchi Grant/research support from: TT received a research grant from Chugai, CoverLetter and a speaker fee from Astellas, Chugai, Eisai, Mitsubishi-Tanabe, Abbvie, Bristol-Myers Squibb, Ayumi, Daiichi Sankyo, Eisai, Takeda, and Asahi-Kasei, Employee of: TT is affiliated with a department that is financially supported by six pharmaceutical companies (Mitsubishi-Tanabe, Chugai, Ayumi, Astellas, Eisai, and Takeda), Hideyuki Shiba: None declared, Yonsu Son: None declared, Hideki Amuro: None declared, Akira Onishi Speakers bureau: AO received a speaker fee from Chugai, Ono Pharmaceutical, Eli Lilly, Mitsubishi-Tanabe, Asahi-Kasei, and Takeda, Kengo Akashi: None declared, Ryota Hara Speakers bureau: RH received a speaker fee from AbbVie, Masaki Katayama: None declared, Keiichi Yamamoto: None declared, Atsushi Kumanogoh Grant/research support from: AK received a research grant and/or speaker fee from Mitsubishi-Tanabe, Chugai, Eisai, Asahi-Kasei, Astellas, Abbvie, Bristol-Myers Squibb, Ono Pharmaceutical, and Pfizer, Speakers bureau: AK received a research grant and/or speaker fee from Mitsubishi-Tanabe, Chugai, Eisai, Asahi-Kasei, Astellas, Abbvie, Bristol-Myers Squibb, Ono Pharmaceutical, and Pfizer, Makoto Hirao Speakers bureau: MHirao received a speaker fee from Astellas, Ono Pharmaceutical, Eli Lilly, Mitsubishi-Tanabe, Pfizer, Ayumi, and Takeda