

Background: Guselkumab (GUS), an interleukin-23 inhibitor, was efficacious in reducing signs and symptoms of active psoriatic arthritis (PsA) in patients (pts) in two phase 3 trials (DISCOVER-1 and DISCOVER-2).
Objectives: To evaluate the efficacy of GUS in PsA pts with imaging-confirmed axial involvement consistent with sacroiliitis in DISCOVER-1&2.
Methods: In DISCOVER-1, 381 pts with active PsA (≥ 3 swollen joints, ≥ 3 tender joints; C-reactive protein ≥ 0.3mg/dL despite standard therapies) and in DISCOVER-2, 739 pts with active PsA (≥ 5 swollen joints, ≥ 5 tender joints, CRP ≥ 0.6mg/dL despite standard therapies) were randomized 1:1:1 to GUS 100mg Q4W, GUS 100mg Q8W (Wk0, Wk4, then Q8W), or PBO. This analysis included pts with sacroiliitis at baseline who had either documented imaging confirmation of sacroiliitis in the past or pelvic X-ray confirmation of sacroiliitis at screening (pooled data from DISCOVER-1&2) based on investigators’ judgment of presence/absence of sacroiliitis. Efficacy was assessed by BASDAI score, BASDAI50, modified BASDAI (mBASDAI; excludes Q#3), spinal pain (BASDAI Q#2), ASDAS-CRP score, and ASDAS responses of inactive disease (<1.3), major improvement (decrease ≥2.0), and clinically important improvement (decrease ≥1.1). Pts with missing data at wk24 were classified as nonresponders.
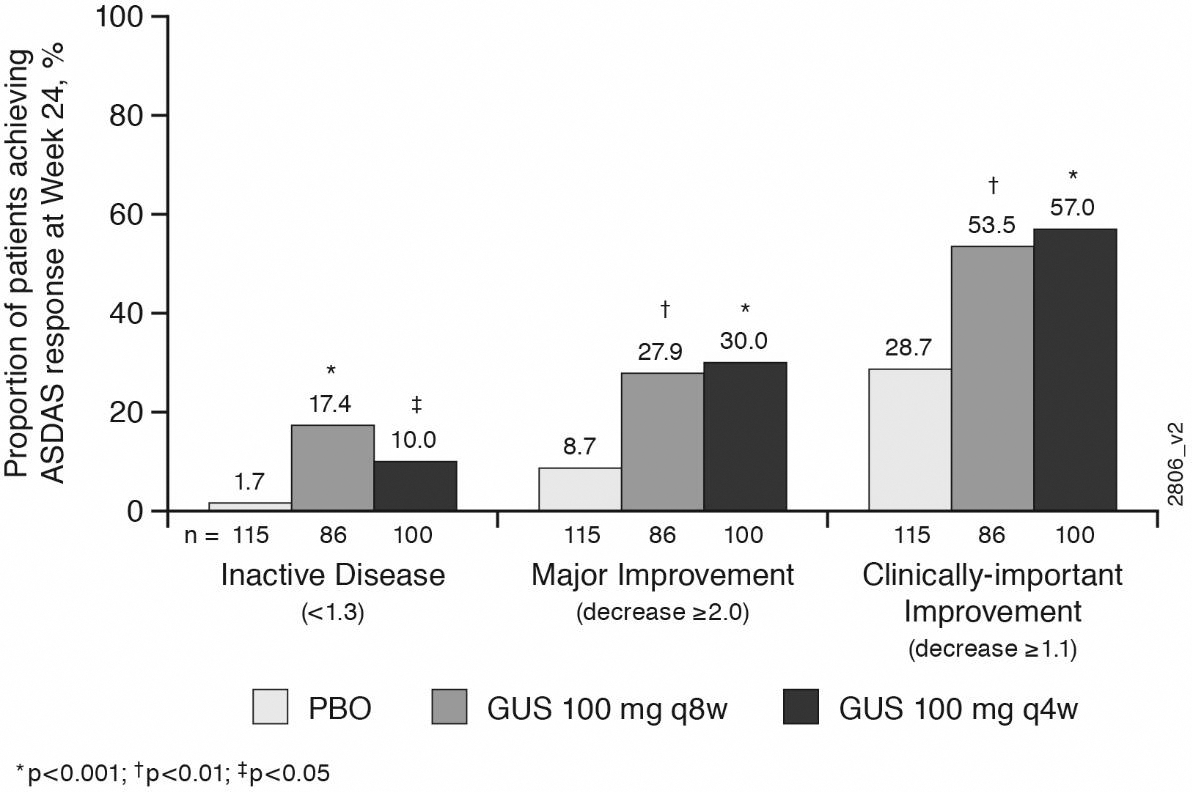
Results: 312 pts presented with axial involvement (PBO, n= 118; GUS q8w, n = 91; GUS q4w, n = 103). The LS mean changes from baseline to wk24 in BASDAI, spinal pain, mBASDAI, and ASDAS-CRP were greater in the two GUS groups vs PBO (Table). Greater proportions of GUS-treated pts achieved BASDAI50 (Table) and ASDAS responses of inactive disease, major improvement, and clinically important improvement (Figure) at wk24 vs PBO.
Conclusion: GUS improved axial symptoms over 24 weeks in active PsA patients with imaging-confirmed sacroiliitis.
Efficacy of GUS in PsA patients with axial involvement at week 24. a
| PBO
| GUS 100 mg every 8 weeks
| GUS100 mg every 4 weeks
|
|
|---|---|---|---|
| LS Mean change in BASDAI | -1.35 | -2.67* | -2.68* |
| LS Mean change in spinal pain b | -1.30 | -2.73* | -2.48* |
| BASDAI50 c , % | 21/110 (19.1%) | 34/84 (40.5%)** | 36/95 (37.9%)** |
| LS Mean change in modified BASDAI d | -1.13 | -2.16* | -2.18* |
| LS Mean change in ASDAS-CRP | -0.71 | -1.43* | -1.46* |
a Pts with axial involvement consistent with sacroiliitis at baseline and either a history of imaging confirmation or pelvic X-ray at screening (pooled data from DISCOVER-1 & 2)
b Question 2 of the BASDAI.
c Pts with BASDAI > 0 at baseline.
d Excludes question 3 of the BASDAI.
Unadjusted p-values as noted: *p < 0.001, ** p < 0.01
Acknowledgments: None
Disclosure of Interests: Philip Helliwell: None declared, Dafna D Gladman Grant/research support from: AbbVie, Amgen Inc., BMS, Celgene Corporation, Janssen, Novartis, Pfizer, UCB – grant/research support, Consultant of: AbbVie, Amgen Inc., BMS, Celgene Corporation, Janssen, Novartis, Pfizer, UCB – consultant, Denis Poddubnyy Grant/research support from: AbbVie, MSD, Novartis, and Pfizer, Consultant of: AbbVie, Bristol-Myers Squibb, Eli Lilly, MSD, Novartis, Pfizer, Roche, UCB, Speakers bureau: AbbVie, Bristol-Myers Squibb, Eli Lilly, MSD, Novartis, Pfizer, Roche, UCB, Philip J Mease Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, Pfizer, Sun Pharma, UCB Pharma, Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, Galapagos, Gilead, Novartis, Pfizer, Sun Pharma, UCB Pharma, Speakers bureau: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Novartis, Pfizer, UCB Pharma, Xenofon Baraliakos Grant/research support from: Grant/research support from: AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB and Werfen, Consultant of: AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB and Werfen, Speakers bureau: AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB and Werfen, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Elizabeth C Hsia Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Xie L Xu Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Shihong Sheng Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Prasheen Agarwal Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Bei Zhou Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Soumya D Chakravarty Shareholder of: Johnson & Johnson, Employee of: Janssen Scientific Affairs, LLC, May Shawi Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Chetan Karyekar Shareholder of: Johnson & Johnson, Consultant of: Janssen, Employee of: Janssen Global Services, LLC. Previously, Novartis, Bristol-Myers Squibb, and Abbott Labs., Atul Deodhar Grant/research support from: AbbVie, Eli Lilly, GSK, Novartis, Pfizer, UCB, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myer Squibb (BMS), Eli Lilly, GSK, Janssen, Novartis, Pfizer, UCB, Speakers bureau: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myer Squibb (BMS), Eli Lilly, GSK, Janssen, Novartis, Pfizer, UCB, Désirée van der Heijde Consultant of: AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli-Lilly, Galapagos, Gilead Sciences, Inc., Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB Pharma; Director of Imaging Rheumatology BV