

Background: Axial spondylarthritis (axSpA) patients treated with a tumour necrosis factor inhibitor (TNFi) may receive a concomitant conventional synthetic disease-modifying anti-rheumatic drug (csDMARD), although the value of combination therapy remains unclear.
Objectives: Describe the proportion and phenotype of patients with axSpA initiating their first TNFi as monotherapy compared to TNFi+csDMARD combination therapy, and to compare the 1-year TNFi retention between the two groups.
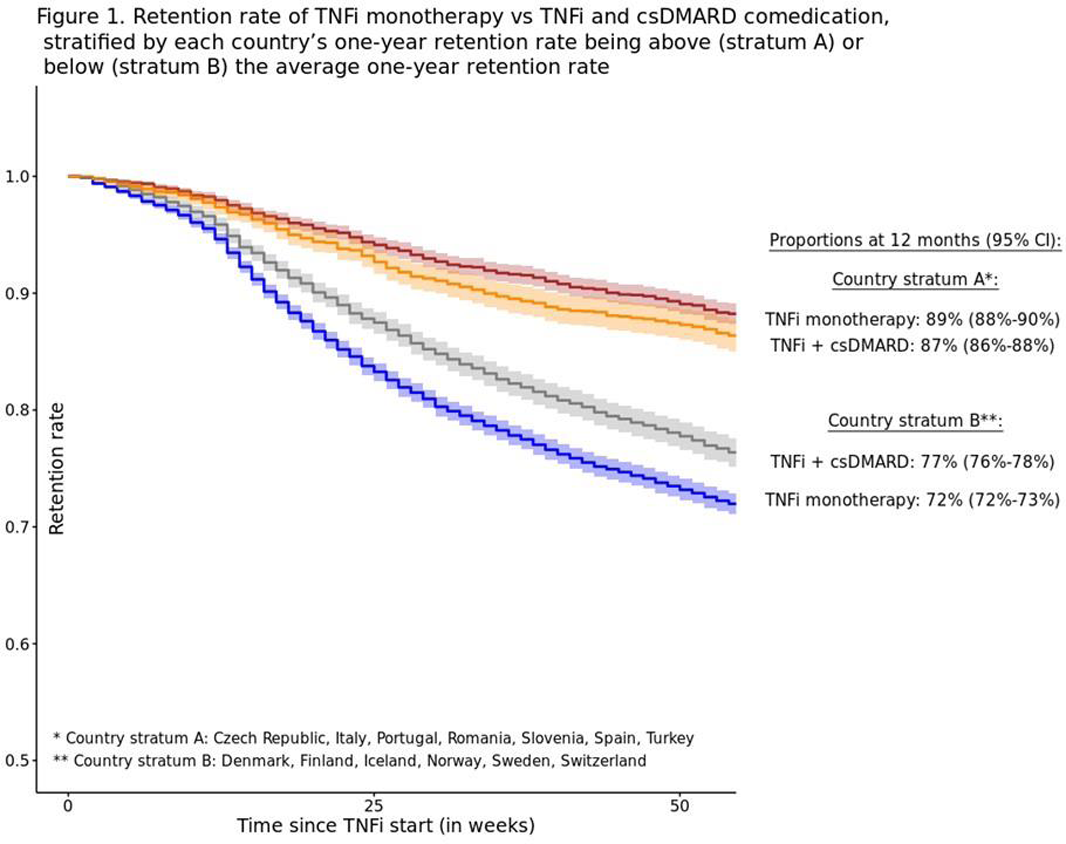
Methods: Data from 13 European registries was collected. Two exposure treatment groups were defined: TNFi monotherapy at baseline (=TNFi start date) and TNFi+csDMARD combination therapy. TNFi retention rates were assessed with Kaplan-Meier curves for each country and combined. Hazard ratios (HR, 95% CI) for discontinuing the TNFi were obtained with Cox models: (i) crude; adjusted for (ii) country, and (iii) country, sex, age, calendar year, disease duration and BASDAI. Participating countries were dichotomized into two strata, depending on their 1-year retention rate being above (stratum A) or below (stratum B) the average retention rate across all countries.
Results: 22,196 axSpA patients were included with 34% on TNFi+csDMARD combination therapy. Baseline characteristics are presented in
| Baseline characteristics | All patients
| Country stratum A | Country stratum B | ||
|---|---|---|---|---|---|
| TNFi mono
| csDMARD + TNFi
| TNFi mono
| csDMARD + TNFi
|
||
| Age (years), mean (SD) | 42.6 (12.5) | 43.4 (12.0) | 42.8 (12.2) | 41.6 (12.7) | 43.7 (12.7) |
| Females, % | 41.1 | 37.7 | 38.2 | 42.0 | 44.2 |
| Disease duration (yrs), mean (SD) | 5.7 (8.0) | 6.2 (7.7) | 6.7 (7.4) | 4.9 (8.2) | 6.1 (8.2) |
| Enthesitis, % | 50.3 | 16.7 | 33.9 | 57.8 | 59.7 |
| SJC-28, median (IQR) | 0 (0-1) | 0 (0-0) | 0 (0-2) | 0 (0-0) | 0 (0-2) |
| VAS pain (0-100), mean (SD) | 60.9 (24.5) | 63.3 (26.5) | 67.8 (23.3) | 60.2 (23.4) | 57.2 (24.3) |
| CRP (mg/L), median (IQR) | 8 (3-20) | 7.8 (2-20) | 18 (6.7-32.6) | 6.0 (2.7-15) | 8.0 (3-22) |
| BASDAI (0-10), mean (SD) | 5.7 (2.1) | 5.7 (2.2) | 6.2 (2.1) | 5.6 (2.0) | 5.4 (2.2) |
| BASFI (0-10), mean (SD) | 4.4 (2.5) | 4.4 (2.6) | 4.9 (2.5) | 4.3 (2.4) | 4.2 (2.9) |
| ASDAS, mean (SD) | 3.5 (1.1) | 3.7 (1.0) | 4.0 (1.0) | 3.3 (1.0) | 3.3 (1.1) |
| On Infliximab, % | 25.7 | 21 | 22 | 24 | 36 |
| Baseline csDMARD use, % | |||||
| -Methotrexate | 0 | 45 | 0 | 63 | |
| -Sulfasalazine | 0 | 68 | 0 | 33 | |
| -Leflunomide | 0 | 8 | 0 | 1 | |
Conclusion: Considerable differences were observed across countries in the use of combination therapy and TNFi retention in axSpA patients. The overall 1-year TNFi retention was higher with csDMARD co-therapy compared to TNFi monotherapy. TNFi monotherapy had a 12-13% higher risk of treatment discontinuation.
Acknowledgments: Novartis Pharma AG and IQVIA
MN and BD participated equally
Disclosure of Interests: Michael Nissen Grant/research support from: Abbvie, Consultant of: Novartis, Lilly, Abbvie, Celgene and Pfizer, Speakers bureau: Novartis, Lilly, Abbvie, Celgene and Pfizer, Bénédicte Delcoigne: None declared, Daniela Di Giuseppe: None declared, Lennart T.H. Jacobsson Consultant of: AbbVie, Eli Lilly, Janssen, Novartis and Pfizer, Karen Fagerli: None declared, Anne Gitte Loft Grant/research support from: Novartis, Consultant of: AbbVie, MSD, Novartis, Pfizer and UCB, Speakers bureau: AbbVie, MSD, Novartis, Pfizer and UCB, Adrian Ciurea Consultant of: Consulting and/or speaking fees from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Merck Sharp & Dohme, Novartis and Pfizer., Dan Nordström Consultant of: Abbvie, Celgene, Lilly, Novartis, Pfizer, Roche and UCB., Speakers bureau: Abbvie, Celgene, Lilly, Novartis, Pfizer, Roche and UCB., Ziga Rotar Consultant of: Speaker and consulting fees from Abbvie, Amgen, Biogen, Eli Lilly, Medis, MSD, Novartis, Pfizer, Roche, Sanofi., Speakers bureau: Speaker and consulting fees from Abbvie, Amgen, Biogen, Eli Lilly, Medis, MSD, Novartis, Pfizer, Roche, Sanofi., Florenzo Iannone Consultant of: Speaker and consulting fees from AbbVie, Eli Lilly, Novartis, Pfizer, Roche, Sanofi, UCB, MSD, Speakers bureau: Speaker and consulting fees from AbbVie, Eli Lilly, Novartis, Pfizer, Roche, Sanofi, UCB, MSD, Maria Jose Santos Speakers bureau: Novartis and Pfizer, Manuel Pombo-Suarez Consultant of: Janssen, Lilly, MSD and Sanofi., Speakers bureau: Janssen, Lilly, MSD and Sanofi., Björn Gudbjornsson Speakers bureau: Novartis and Amgen, Heřman Mann: None declared, Nurullah Akkoc: None declared, Catalin Codreanu Consultant of: Speaker and consulting fees from AbbVie, Accord Healthcare, Alfasigma, Egis, Eli Lilly, Ewopharma, Genesis, Mylan, Novartis, Pfizer, Roche, Sandoz, UCB, Speakers bureau: Speaker and consulting fees from AbbVie, Accord Healthcare, Alfasigma, Egis, Eli Lilly, Ewopharma, Genesis, Mylan, Novartis, Pfizer, Roche, Sandoz, UCB, Irene van der Horst-Bruinsma Grant/research support from: AbbVie, Novartis, Eli Lilly, Bristol-Myers Squibb, MSD, Pfizer, UCB Pharma, Consultant of: AbbVie, Novartis, Eli Lilly, Bristol-Myers Squibb, MSD, Pfizer, UCB Pharma, Brigitte Michelsen: None declared, Gary Macfarlane: None declared, Merete L. Hetland Grant/research support from: BMS, MSD, AbbVie, Roche, Novartis, Biogen and Pfizer, Consultant of: Eli Lilly, Speakers bureau: Orion Pharma, Biogen, Pfizer, CellTrion, Merck and Samsung Bioepis, Matija Tomsic: None declared, Burkhard Moeller: None declared, Pedro Ávila-Ribeiro Grant/research support from: Novartis, Carlos Sánchez-Piedra: None declared, Heikki Relas Grant/research support from: Abbvie., Consultant of: Abbvie, Celgene, and Pfizer., Speakers bureau: Abbvie, Celgene, and Pfizer., Arni Jon Geirsson: None declared, Lucie Nekvindova: None declared, Gozde Yildirim Cetin Speakers bureau: AbbVie, Novartis, Pfizer, Roche, UCB, MSD, Ruxandra Ionescu Consultant of: Consulting fees from Abbvie, Eli-Lilly, Novartis, Pfizer, Roche, Sandoz, Speakers bureau: Consulting and speaker fees from Abbvie, Eli-Lilly, Novartis, Pfizer, Roche, Sandoz, Niels Steen Krogh: None declared, Johan Askling Grant/research support from: JA acts or has acted as PI for agreements between Karolinska Institutet and the following entities, mainly in the context of the ARTIS national safety monitoring programme of immunomodulators in rheumatology: Abbvie, BMS, Eli Lilly, Merck, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB Pharma, Bente Glintborg Grant/research support from: Grants from Pfizer, Biogen and Abbvie, Ulf Lindström: None declared