

Background: Interstitial Lung Disease (ILD) is a severe complication of Rheumatoid Arthritis (RA). Several conventional disease-modifying anti-rheumatic drugs (cDMARDs) and biologic (b) DMARDs may induce or impaired ILD-RA. Abatacept (ABA) may be useful in ILD-RA (1).
Objectives: To assess the efficacy and safety of ABA in a large series of ILD-RA for a long-term follow-up.
Methods: Multicenter open-level study of ILD-RA treated with at least 1 dose of ABA. ILD was diagnosed by high-resolution computed tomography (HRTC). We study these outcomes: a) 1-point change Modied Medical Research Council (MMRC); b) forced vital capacity (FVC) and/or DLCO improvement or decline ≥10%; c) change in HRCT, d) change in DAS28. e) Prednisone dose. Values were collected at 0, 3, 6, 12 and then every 12 months.
Results: We studied 263 patients (150 women/113 men) (mean age;64.6±10 years), with ILD-RA. At ABA-onset they were smokers or exsmoker (53.8%), positive APCC (88.6%), median [IQR] duration of ILD of 12 [3-41.25] months, mean DLCO (65.7±18.3) and FVC (85.9±21.8).
The ILD-pattern were usual interstitial pneumonia (UIP) (40.3%), non-specific interstitial pneumonia (NSIP) (31.9%) and others (27.8%).
ABA was prescribed at standard subcutaneous (125 mg/w) in 196 (74.5%) or intravenously (10 mg/kg/4 w) in 67 (25.5%); in monotherapy (n=111) or combined with cDMARDs (n=152); especially leflunomide (n=55), MTX (n=46), or antimarials (n=21).
After a mean follow-up of 22.7±19.7 months most outcomes remain stable (Figure). Moreover, DAS28 improved from 4.5±1.5 to 3.1±1.3; prednisone dose reduced from a median 7.5 [5-10] to 5 mg [5-7.5] and retention rate was 76.4%. The main adverse effects were serious infections (n=28), neoplasia (n=3), serious infusion reaction (n=1) and myocardial infarction (n=1).
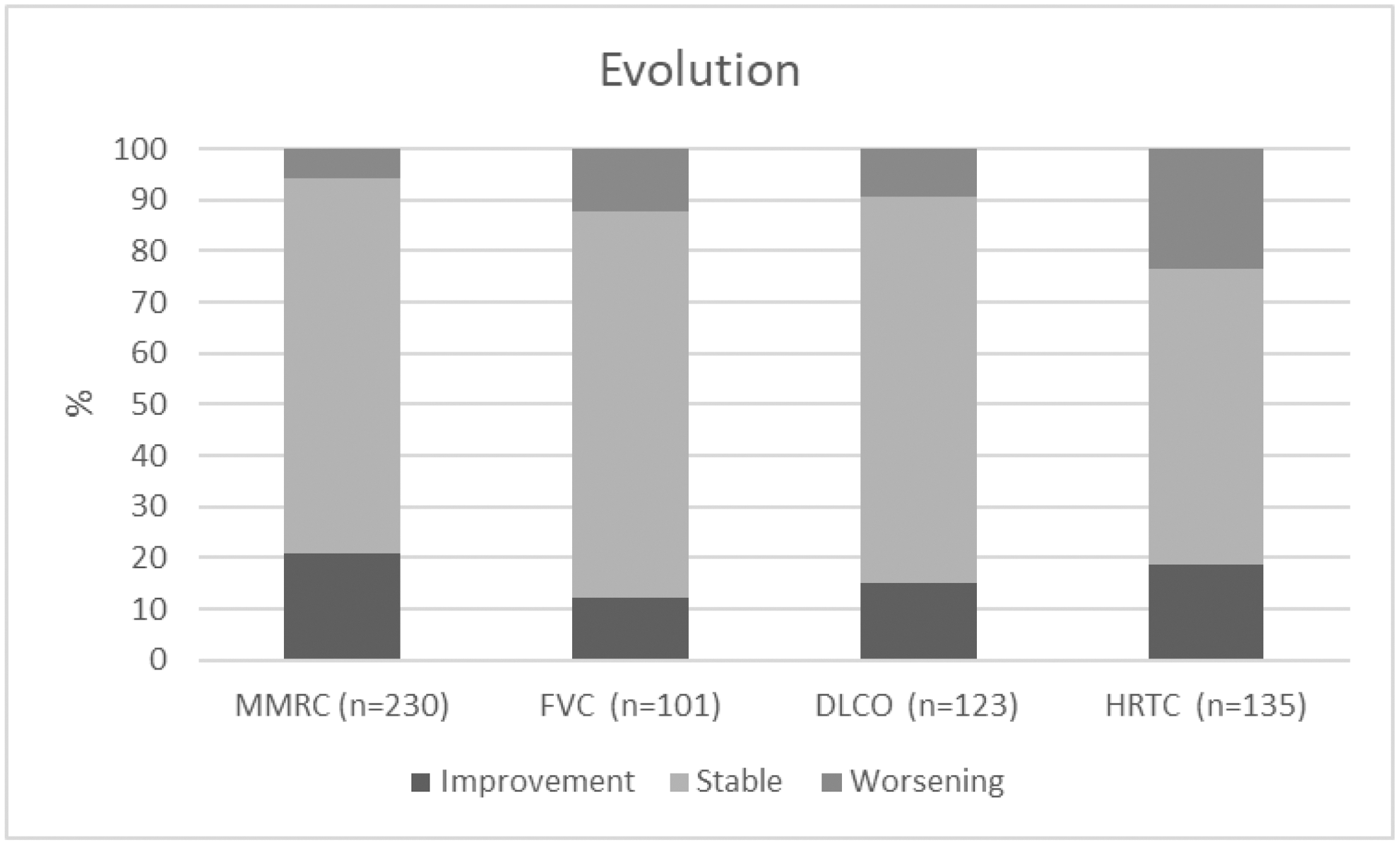
Conclusion: ABA seems effective and relatively safe in ILD-RA.
REFERENCES:
[1]Fernández-Díaz C et al. Semin Arthritis Rheum. 2018; 48:22-27
Disclosure of Interests: Carlos Fernández-Díaz Speakers bureau: Brystol Meyers Squibb, Santos Castañeda: None declared, Rafael Melero: None declared, J. Loricera: None declared, Francisco Ortiz-Sanjuán: None declared, A. Juan-Mas: None declared, Carmen Carrasco-Cubero Speakers bureau: Janssen, MSD, AbbVie, Novartis, Bristol Myers Squibb, and Celgene, S, Rodriguéz-Muguruza: None declared, S. Rodrigez -Garcia: None declared, R. Castellanos-Moreira: None declared, RAQUEL ALMODOVAR Speakers bureau: Abbvie, Celgene, Janssen, Lilly, Novartis, Pfizer.
CLARA AGUILERA CROS: None declared, Ignacio Villa-Blanco Consultant of: UCB, Speakers bureau: Novartis, MSD, Lilly, Sergi Ordoñez: None declared, Susana Romero-Yuste: None declared, C. Ojeda-Garcia: None declared, Manuel Moreno: None declared, Gemma Bonilla: None declared, I. Hernández-Rodriguez: None declared, Mireia Lopez Corbeto: None declared, José Luis Andréu Sánchez: None declared, Trinidad Pérez Sandoval: None declared, Alejandra López Robles: None declared, Patricia Carreira Grant/research support from: Actelion, Roche, MSD, Consultant of: GlaxoSmithKline, VivaCell Biotechnology, Emerald Health Pharmaceuticals, Boehringer Ingelheim, Roche, Speakers bureau: Actelion, GlaxoSmithKline, Roche, Natalia Mena-Vázquez: None declared, C. Peralta-Ginés: None declared, ANA URRUTICOECHEA-ARANA: None declared, Luis Marcelino Arboleya Rodríguez: None declared, J. Narváez: None declared, DESEADA PALMA SANCHEZ: None declared, Olga Maiz-Alonso: None declared, J. Fernández-Leroy: None declared, I. Cabezas-Rodriguez: None declared, Ivan Castellví Consultant of: Boehringer Ingelheim, Actelion, Kern Pharma, Speakers bureau: Boehringer Ingelheim, Actelion, Bristol-Myers Squibb, Roche, A. Ruibal-Escribano: None declared, JR De Dios-Jiménez Aberásturi: None declared, Paloma Vela-Casasempere: None declared, C. González-Montagut Gómez: None declared, J M Blanco: None declared, Noelia Alvarez-Rivas: None declared, N. Del-Val: None declared, M. Rodíguez-Gómez: None declared, Eva Salgado-Pérez: None declared, Carlos Fernández-López: None declared, E.C. Cervantes Pérez: None declared, A. Devicente-DelMas: None declared, Blanca Garcia-Magallon Consultant of: MSD, Speakers bureau: Pfizer, Amgen, Celgene, MSD, Cristina Hidalgo: None declared, Sabela Fernández: None declared, Edilia García-Fernández: None declared, R. López-Sánchez: None declared, S. Castro: None declared, P. Morales-Garrido: None declared, Andrea García-Valle: None declared, Rosa Expósito: None declared, L. Exposito-Perez: None declared, Lorena Pérez Albaladejo: None declared, Ángel García-Aparicio: None declared, Miguel A González-Gay Grant/research support from: Pfizer, Abbvie, MSD, Speakers bureau: Pfizer, Abbvie, MSD, Ricardo Blanco Grant/research support from: AbbVie, MSD, and Roche, Speakers bureau: AbbVie, Pfizer, Roche, Bristol-Myers, Janssen, and MSD