

Background: We have previously observed in RA patients that central nervous system (CNS) response to compression of a painful joint, measured using functional MRI (fMRI) of the brain as the number of blood oxygen level dependent (BOLD) signal positive voxels, is rapidly ameliorated, much earlier than any clinical response with anti-TNF treatment and a high baseline CNS pain response could predict better response to certolizumab pegol (CZP) treatment. Pre-CePRA was designed and conducted to test this effect in a randomized, placebo controlled trial of CZP and showed an incremental linear trend of DAS28 low disease activity (LDA) across study groups treated with placebo, and two CZP arms stratified as low or high pre-treatment CNS pain response.
Objectives: To explore and describe pre-treatment CNS pain response associations with post treatment course of RA disease activity components and patient-physician discrepancy in global disease assessment.
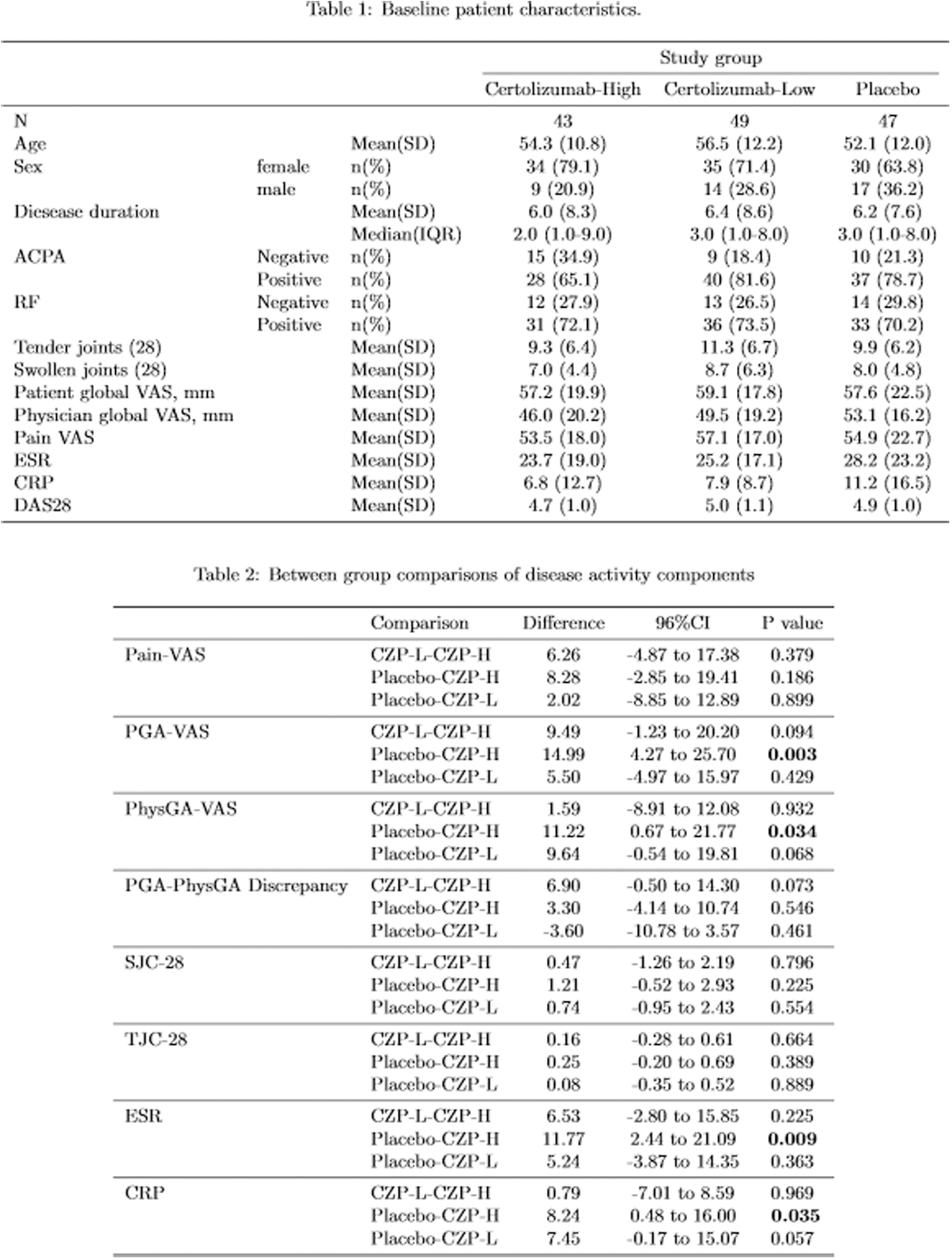
Methods: Patients fulfilling the 2010 ACR/EULAR classification criteria with moderate-severe disease activity (DAS-28>3.2) under stable DMARD treatment were recruited. Patients underwent an fMRI scan, stratified by a whole-brain BOLD positive voxel count threshold of 700 units and randomized to treatment with CZP or placebo in a 2:1 ratio. We descriptively assessed components of RA disease activity (Table 1 + 2). We summarized the mean results and 95% confidence intervals of these measurements at study timepoints and compared the 3 study groups at week 12 using one-way ANOVA and post-hoc Tukey tests.
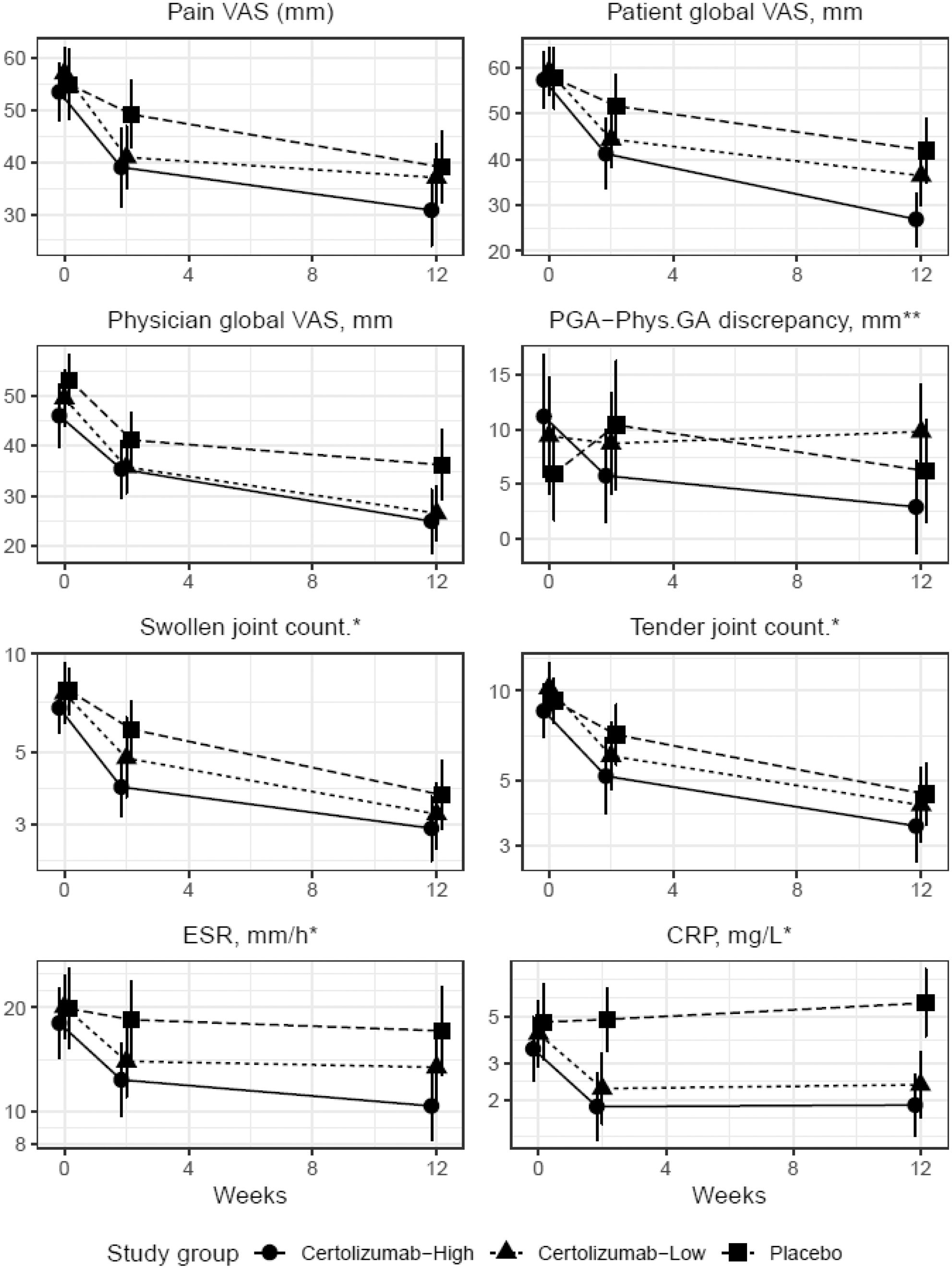
Results: 156 eligible patients were screened and 139 (99 females, 71%) patients with moderate-high disease activity were randomized. ANOVA and pairwise comparisons showed that PGA-VAS improvement was larger in the CZP-H group whereas more similar to that in placebo in the CZP-L group. PhysGA-VAS however was similarly reduced in both CZP groups. Patients in the CZP-L group constantly rated their pain numerically higher than physicians whereas in the CZP-H group an initially higher discrepancy numerically reduced over time.
Conclusion: These results suggest that improved patient global disease activity assessment could be the main driver of improved DAS-28 LDA rates with CZP treatment in patients with a high CNS pain response. Our findings indicate a potential role of fMRI imaging of the brain to further understand disease activity perception in RA patients.
Course of disease activity components through trial timepoints. *indicates log-transformed y axis. *#x002A; Discrepancy equals Patient global minus physician global assessment.
Disclosure of Interests: Hannah Schenker: None declared, Jürgen Rech Consultant of: BMS, Celgene, Novartis, Roche, Chugai, Speakers bureau: AbbVie, Biogen, BMS, Celgene, MSD, Novartis, Roche, Chugai, Pfizer, Lilly, Koray Tascilar: None declared, Melanie Hagen: None declared, Verena Schönau: None declared, Marina Sergeeva: None declared, Mageshwar Selvakumar: None declared, Laura Konerth: None declared, Jutta Prade: None declared, Sandra Strobelt: None declared, Larissa Valor: None declared, Axel Hueber Grant/research support from: Novartis, Lilly, Pfizer, EIT Health, EU-IMI, DFG, Universität Erlangen (EFI), Consultant of: Abbvie, BMS, Celgene, Gilead, GSK, Lilly, Novartis, Speakers bureau: GSK, Lilly, Novartis, David Simon Grant/research support from: Else Kröner-Memorial Scholarship, Novartis, Consultant of: Novartis, Lilly, Arnd Kleyer Consultant of: Lilly, Gilead, Novartis,Abbvie, Speakers bureau: Novartis, Lilly, Frank Behrens Grant/research support from: Abbvie, Pfizer, Roche, Chugai, Janssen, Consultant of: Abbvie, Pfizer, Roche, Chugai, UCB, BMS, Celgene, MSD, Novartis, Biotest, Janssen, Genzyme, Lilly; Boehringer; Sandoz, Speakers bureau: Abbvie, Pfizer, Roche, Chugai, UCB, BMS, Celgene, MSD, Novartis, Biotest, Janssen, Genzyme, Lilly; Boehringer; Sandoz, José Antonio P. da Silva Grant/research support from: Pfizer, Abbvie, Consultant of: Pfizer, AbbVie, Roche, Lilly, Novartis, Christoph Baerwald Consultant of: CGB received speaker or consulting fees from AbbVie, Paid instructor for: CGB received speaker or consulting fees from AbbVie, Speakers bureau: CGB received speaker or consulting fees from AbbVie, Stephanie Finzel: None declared, Reinhard Voll: None declared, Eugen Feist Consultant of: Novartis, Roche, Sobi, Lilly, Pfizer, Abbvie, BMS, MSD, Sanofi, Speakers bureau: Novartis, Roche, Sobi, Lilly, Pfizer, Abbvie, BMS, MSD, Sanofi, Arnd Doerfler: None declared, Nemanja Damjanov Grant/research support from: from AbbVie, Pfizer, and Roche, Consultant of: AbbVie, Gedeon Richter, Merck, Novartis, Pfizer, and Roche, Speakers bureau: AbbVie, Gedeon Richter, Merck, Novartis, Pfizer, and Roche, Andreas Hess: None declared, Georg Schett Speakers bureau: AbbVie, BMS, Celgene, Janssen, Eli Lilly, Novartis, Roche and UCB