

Background: Tofacitinib is an oral JAK inhibitor for the treatment of psoriatic arthritis (PsA).
Objectives: To assess tofacitinib 5 mg BID as monotherapy after methotrexate (MTX) withdrawal vs with continued background MTX in patients (pts) with PsA.
Methods: OPAL Balance (NCT01976364) was an open-label (OL) long-term extension (LTE) study of tofacitinib in pts with PsA who participated in Phase (P)3 studies (OPAL Broaden, NCT01877668; OPAL Beyond, NCT01882439). Pts who completed ≥24 months’ tofacitinib treatment in the LTE (stable 5 mg BID for ≥3 months) and were receiving oral MTX (7.5–20 mg/week; stable for ≥4 weeks) entered the multicentre, 12-month, double-blind, MTX withdrawal substudy. Pts remained on OL tofacitinib 5 mg BID and were randomised 1:1 to receive placebo (tofacitinib monotherapy, ie, blinded MTX withdrawal) or MTX (tofacitinib + MTX; same stable doses). Primary endpoints were changes from substudy baseline (Δ) in PASDAS and HAQ-DI at Month (M)6. Secondary efficacy endpoints were assessed at all time points. Safety was assessed throughout the substudy.
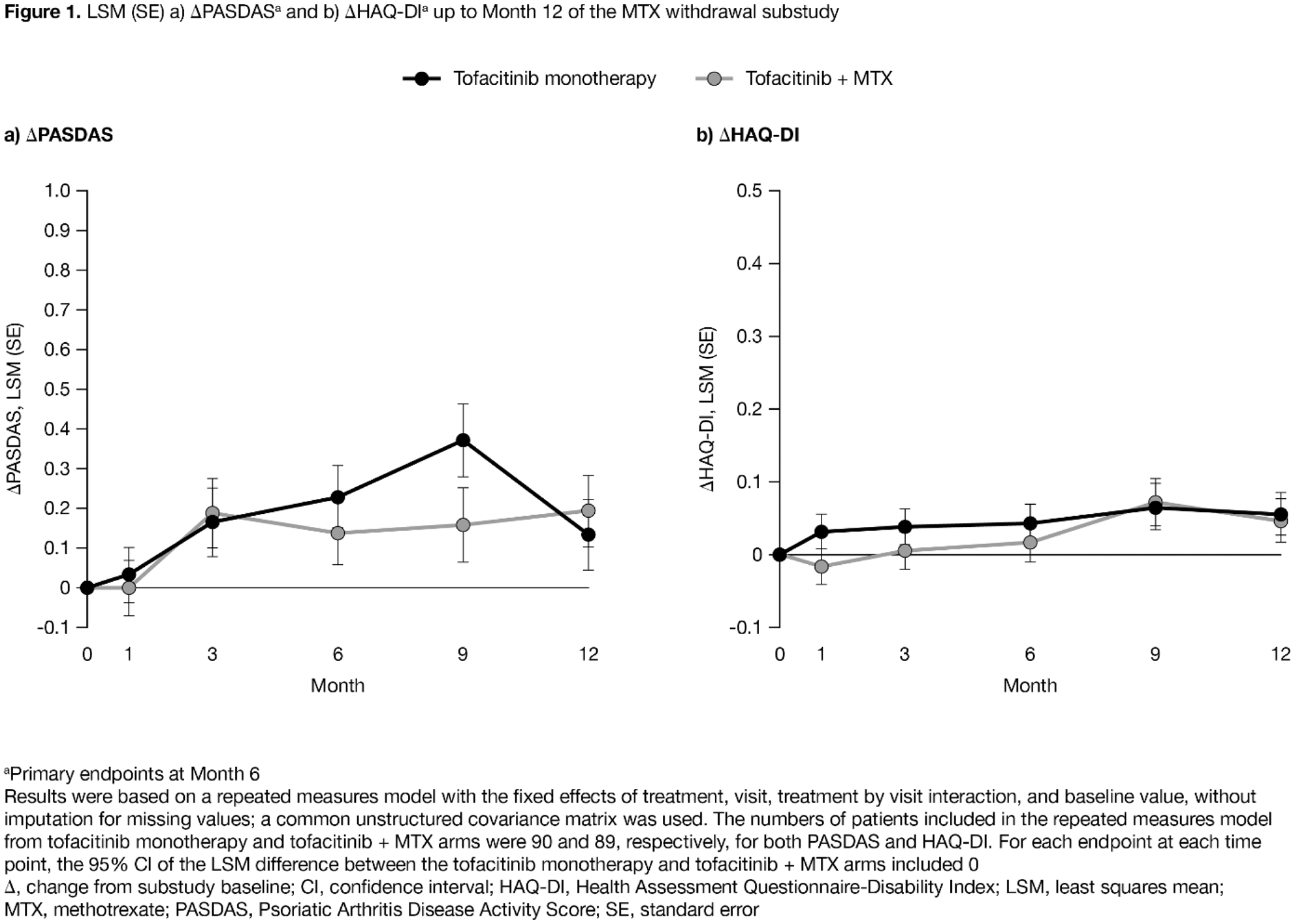
Results: Of 180 pts randomised, 179 were treated (tofacitinib monotherapy n=90; tofacitinib + MTX n=89). Pt characteristics were similar between treatment arms. At M6, least squares mean (LSM) (standard error [SE]) ΔPASDAS was 0.229 (0.079) for tofacitinib monotherapy and 0.138 (0.081) for tofacitinib + MTX, and LSM (SE) ΔHAQ-DI was 0.043 (0.027) and 0.017 (0.028), respectively (
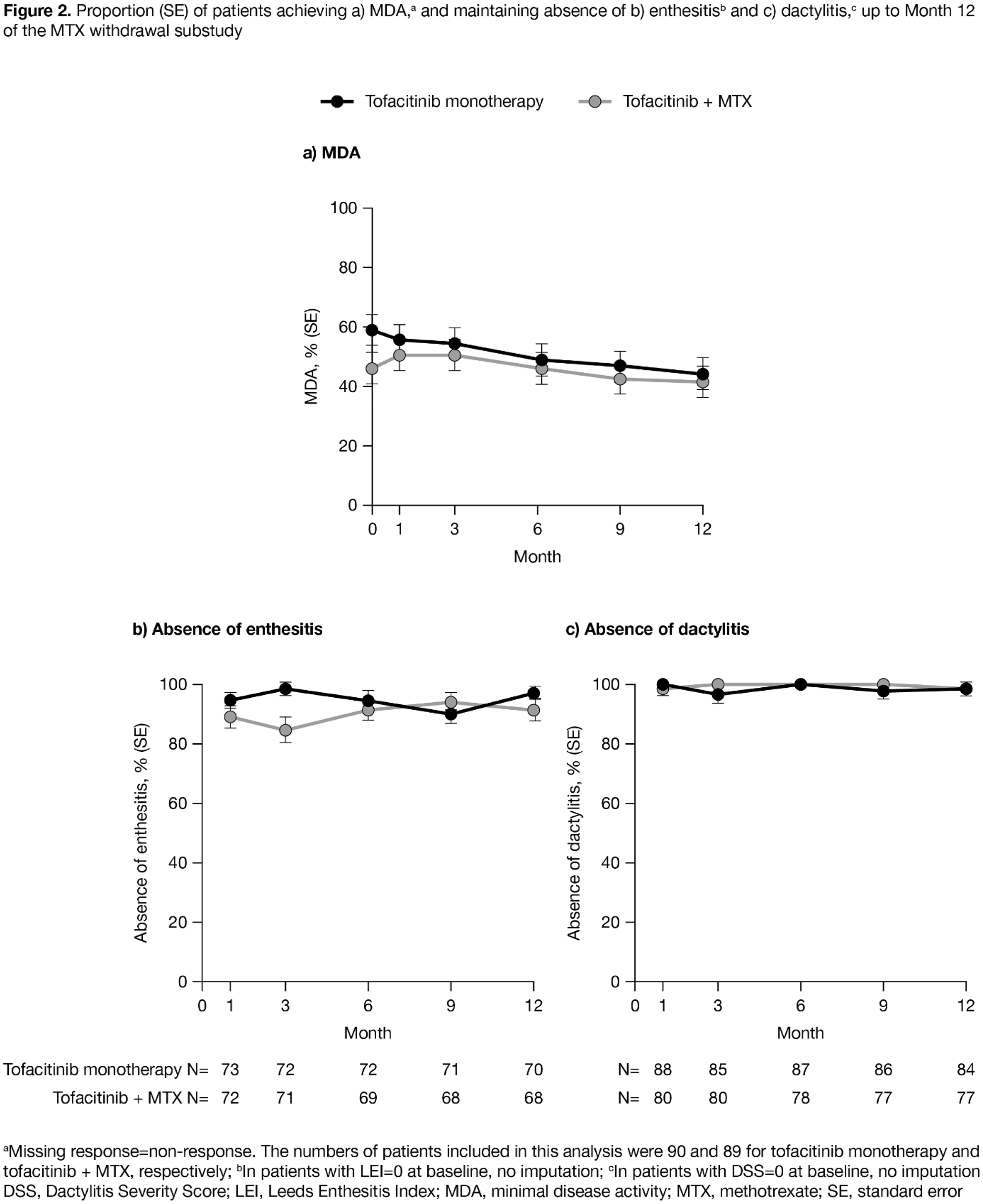
Conclusion: No clinically meaningful differences in efficacy and safety were observed in PsA pts who received OL tofacitinib 5 mg BID as monotherapy after MTX withdrawal vs with continued MTX. Safety was consistent with previous P3 studies. The substudy was an estimation study and not powered for hypothesis testing.
Safety outcomes to Month 12
| Pts with events, n (%) AEs of special interest | Tofacitinib monotherapy N=90 |
Tofacitinib + MTX
|
|---|---|---|
| AE | 43 (47.8) | 41 (46.1) |
| Serious AE | 4 (4.4) | 3 (3.4) |
| Discontinuations due to AE | 3 (3.3) | 4 (4.5) |
| Death | 0 | 0 |
| Herpes zoster (serious/non-serious) | 1 (1.1) | 2 (2.2) |
| Serious infection | 0 | 2 (2.2) |
| Opportunistic infection a | 0 | 1 (1.1) |
| Malignancy (excl. NMSC) a | 1 (1.1) | 1 (1.1) |
| NMSC a | 0 | 0 |
| Major adverse cardiovascular event a | 0 | 0 |
| Venous thromboembolism c | 0 | 0 |
| Arterial thromboembolism c | 1 (1.1) | 0 |
| Gastrointestinal perforation a | 0 | 0 |
| Interstitial lung disease b | 0 | 0 |
| Laboratory parameters d | ||
| ALT ≥3×ULN | 0 | 5 (5.6) |
| ALT (IU/L), mean (SE) | -2.7 (1.6) | 2.5 (1.3) |
| AST ≥3×ULN | 0 | 3 (3.4) |
| AST (IU/L), mean (SE) | -1.5 (1.2) | 1.7 (0.8) |
Reviewed by independent a external/ b internal adjudication committee
c Per Standardised MedDRA Query terms
d Without regard to baseline abnormality
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Christina Viegelmann of CMC Connect and funded by Pfizer Inc.
Disclosure of Interests: Peter Nash Grant/research support from: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Speakers bureau: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Laura C Coates: None declared, Philip J Mease Grant/research support from: Abbott, Amgen, Biogen Idec, BMS, Celgene Corporation, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, UCB – grant/research support, Consultant of: Abbott, Amgen, Biogen Idec, BMS, Celgene Corporation, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, UCB – consultant, Speakers bureau: Abbott, Amgen, Biogen Idec, BMS, Eli Lilly, Genentech, Janssen, Pfizer, UCB – speakers bureau, Alan Kivitz Shareholder of: AbbVie, Amgen, Gilead, GSK, Pfizer Inc, Sanofi, Consultant of: AbbVie, Boehringer Ingelheim,,Flexion, Genzyme, Gilead, Janssen, Novartis, Pfizer Inc, Regeneron, Sanofi, SUN Pharma Advanced Research, UCB, Paid instructor for: Celgene, Genzyme, Horizon, Merck, Novartis, Pfizer, Regeneron, Sanofi, Speakers bureau: AbbVie, Celgene, Flexion, Genzyme, Horizon, Merck, Novartis, Pfizer Inc, Regeneron, Sanofi, Dafna D Gladman Grant/research support from: AbbVie, Amgen Inc., BMS, Celgene Corporation, Janssen, Novartis, Pfizer, UCB – grant/research support, Consultant of: AbbVie, Amgen Inc., BMS, Celgene Corporation, Janssen, Novartis, Pfizer, UCB – consultant, Frank Behrens Grant/research support from: Pfizer, Janssen, Chugai, Celgene, Lilly and Roche, Consultant of: Pfizer, AbbVie, Sanofi, Lilly, Novartis, Genzyme, Boehringer, Janssen, MSD, Celgene, Roche and Chugai, James Cheng-Chung Wei Grant/research support from: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, UCB, Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Eisai, Janssen, Novartis, Pfizer Inc, Sanofi-Aventis, UCB Pharma, Dona Fleishaker Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Joseph Wu Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Cunshan Wang Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Ana Belen Romero Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Lara Fallon Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Ming-Ann Hsu Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Keith Kanik Shareholder of: Pfizer Inc, Employee of: Pfizer Inc