

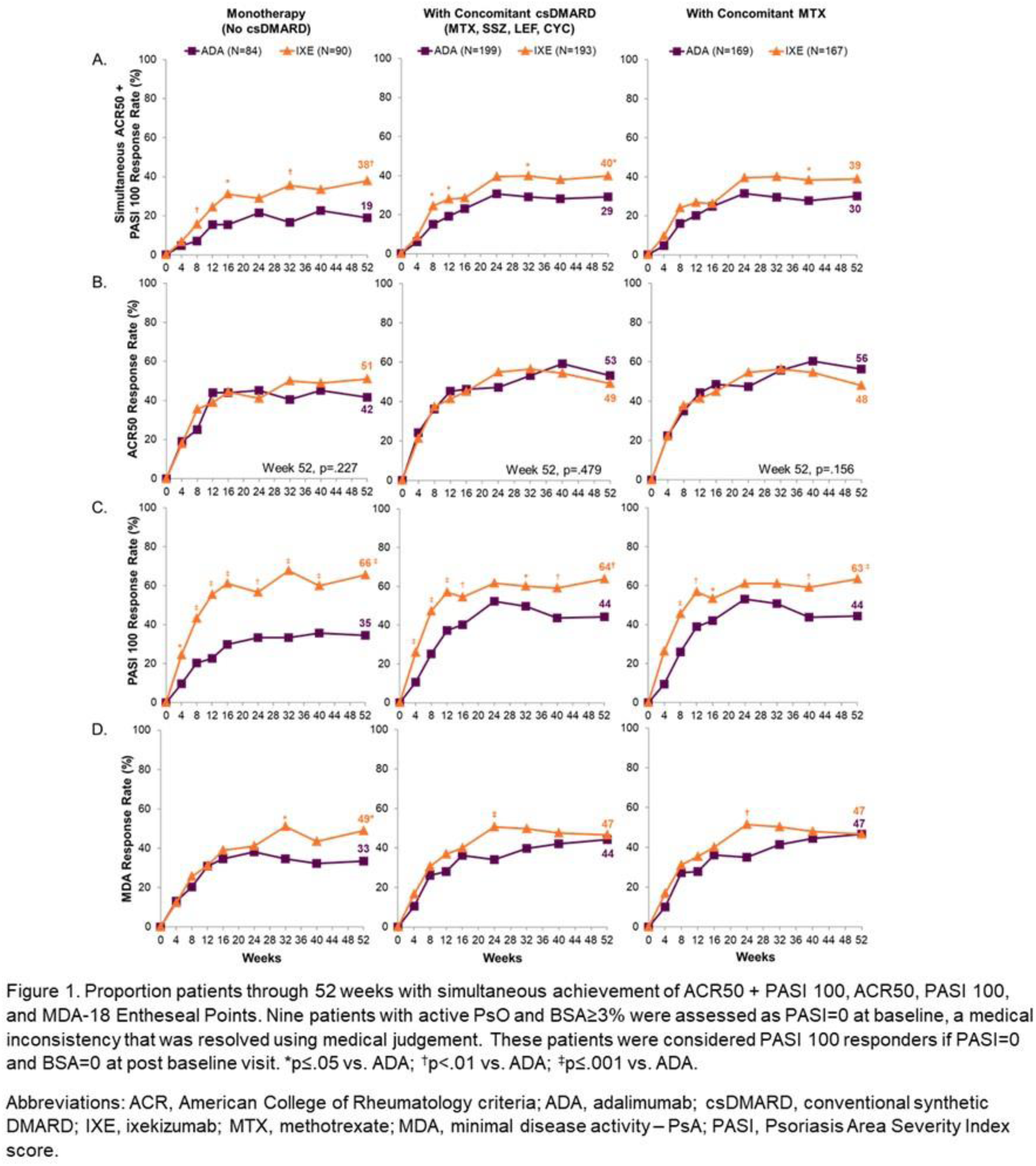
Background: Ixekizumab (IXE), a high-affinity monoclonal antibody selectively targeting IL-17A, was superior to adalimumab (ADA) at Week (Wk) 24 for simultaneous achievement of ACR50 and 100% improvement from baseline in the Psoriasis Area and Severity Index (PASI 100) (primary endpoint) in patients (pts) with active PsA from SPIRIT-H2H 1 . SPIRIT-H2H had two major secondary endpoints and achieved both: noninferiority of IXE to ADA for ACR50 at Wk 24, and superiority of IXE to ADA for PASI 100 at Wk 24.
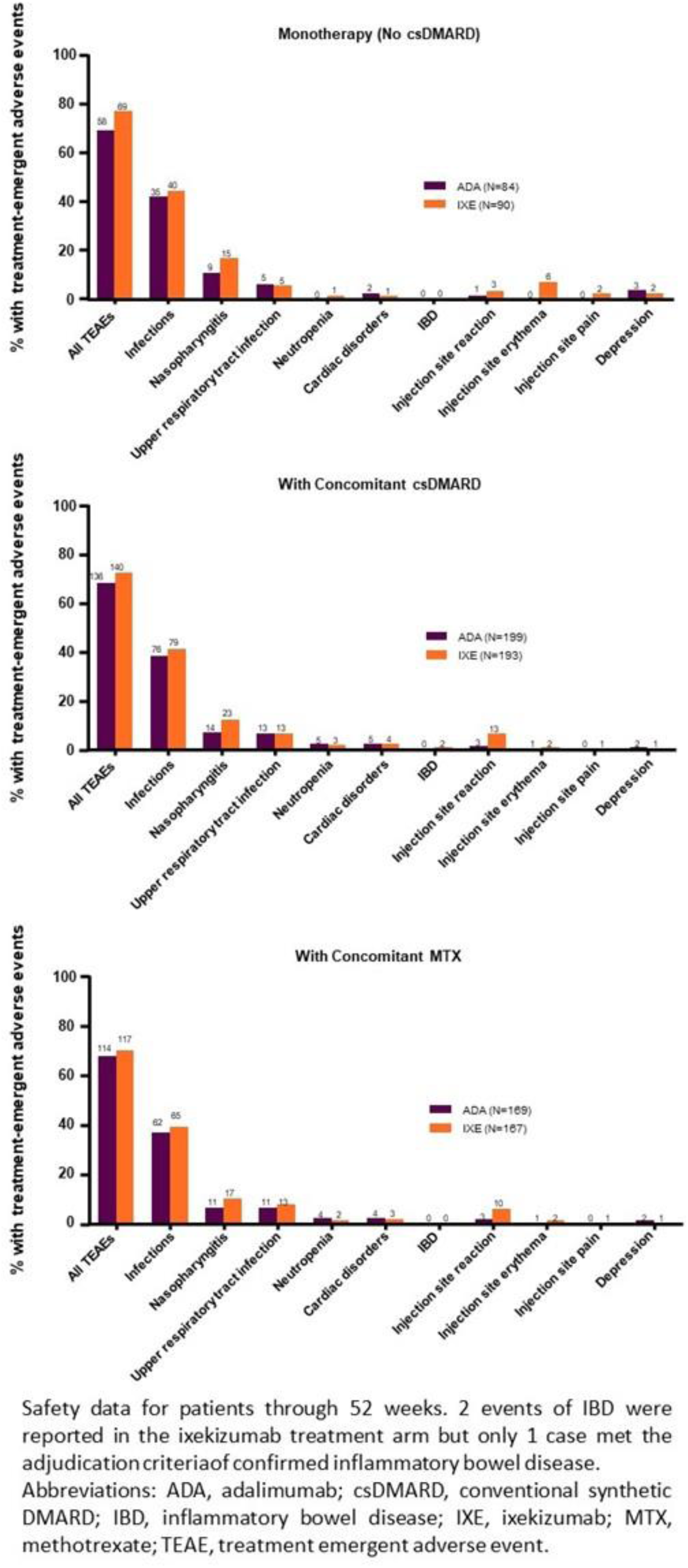
Objectives: To determine how concomitant conventional synthetic DMARD (csDMARD) use affects safety and efficacy of IXE and ADA in prespecified subgroups defined by biologic monotherapy, concomitant MTX use, and concomitant csDMARD use through Wk 52 in SPIRIT-H2H.
Methods: SPIRIT-H2H (NCT03151551) was a 52-week, multicentre, randomised, open-label, assessor-blinded, parallel-group study evaluating the efficacy and safety of IXE versus ADA in adults with PsA and naïve to biologic DMARDs. Patients were required to have active PsA fulfilling Classification for Psoriatic Arthritis (CASPAR) criteria and ≥3/68 tender and ≥3/66 swollen joints, ≥3% plaque psoriasis BSA involvement, no prior treatment with bDMARDs, and with prior inadequate response to ≥1 csDMARD (but not necessarily current treatment with csDMARDs). Randomization (1:1) was stratified by concomitant use of csDMARD and the presence/absence of moderate to severe PsO (baseline: BSA≥10% + PASI≥12, + static Physician’s Global Assessment≥3). Patients (N=566) received IXE/ADA through 52 wks according to the labelled dose dependent on presence/absence of moderate-to-severe PsO. In this prespecified subgroup analysis by presence or absence of csDMARDs, efficacy outcomes through wk 52 were compared between IXE and ADA using logistic regression models and Fisher’s exact tests. Missing data were imputed using non-responder imputation.
Results: At baseline, 167 of 283 IXE-treated patients and 169 of 283 ADA-treated patients had concomitant MTX use. Of these, 9.0% (15/167) and 7.1% (12/169) treated with IXE and ADA, respectively, were taking an additional csDMARD (sulfasalazine, cyclosporine, or leflunomide). A significantly greater proportion of patients on IXE versus ADA achieved the primary endpoint or PASI 100 when used as monotherapy or in combination with csDMARD (
Conclusion: As with prior studies, 2,3 consistent efficacy across multiple PsA disease-specific endpoints was observed with IXE in SPIRIT-H2H, regardless of whether IXE was taken as monotherapy or in combination with MTX or another csDMARD. No unexpected safety signals were found for either agent.
REFERENCES:
[1]Mease et al, Ann Rheum Dis 2020;79:123-31.
[2]Coates et al, RMD Open 2017;3:e000567.
[3]Nash et al, RMD Open 2018;4:e000692.
Disclosure of Interests: Josef S. Smolen Grant/research support from: AbbVie, AstraZeneca, Celgene, Celltrion, Chugai, Eli Lilly, Gilead, ILTOO, Janssen, Novartis-Sandoz, Pfizer Inc, Samsung, Sanofi, Consultant of: AbbVie, AstraZeneca, Celgene, Celltrion, Chugai, Eli Lilly, Gilead, ILTOO, Janssen, Novartis-Sandoz, Pfizer Inc, Samsung, Sanofi, Anthony Sebba Consultant of: Genentech, Gilead, Lilly, Regeneron Pharmaceuticals Inc., Sanofi, Speakers bureau: Lilly, Roche, Sanofi, Eric Ruderman Consultant of: Pfizer, Amanda Gellett Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Christophe Sapin Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Aubrey Trevelin Sprabery Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Soyi Liu Leage Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Sreekumar Pillai Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Paulo Reis Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Peter Nash Grant/research support from: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Speakers bureau: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB