

Background: Systemic Autoinflammatory diseases present with sterile inflammation. NOMID (Neonatal-Onset Multisystem Inflammatory Disease) is caused by gain-of-function mutations in NLRP3 and excess IL-1 production, presents with fever, neutrophilic dermatosis, aseptic meningitis, hearing loss and eye inflammation; CANDLE (Chronic Atypical Neutrophilic Dermatosis, Lipodystrophy and Elevated Temperature) is caused by loss-of-function mutations in proteasome genes that lead to type-1 interferon signaling, characterized by fever, panniculitis, lipodystrophy, cytopenia, systemic and pulmonary hypertension and basal ganglia calcification. IL-1 blockers are approved for NOMID and JAK-inhibitors show efficacy in CANDLE treatment.
Objectives: We used proteomic analysis to compare differentially expressed proteins in active NOMID and CANDLE compared to healthy controls before and after treatment, and whole blood bulk RNA seq to identify the immune cell signatures.
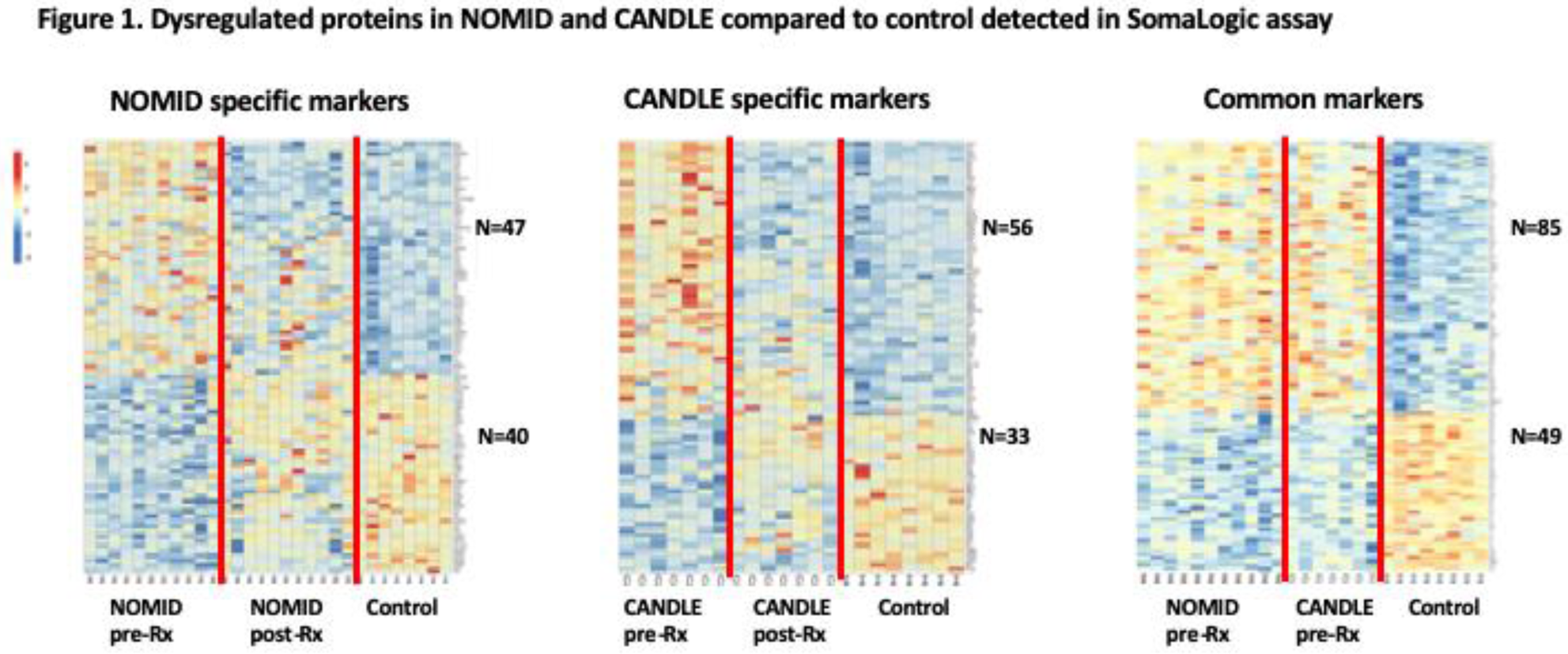
Methods: Serum samples from active NOMID (n=12) and CANDLE (n=7) before and after treatment (
Patient’s characteristics
|
NOMID
|
CANDLE
|
|||
|---|---|---|---|---|
| Age
| 12 (2, 28) | 16 (3, 20) | ||
| Ethnicity
| 80 (20) | 100 (30) | ||
| Genetics |
NLRP3
mutation
| mutations in proteasome component genes
|
||
| Before treatment | After treatment | Before treatment | After treatment | |
| CRP
| 52 (16-110) | 5 (0-23) | 5 (0-101) | 1 (0-4) |
| IFN score
| 0 | NA | 328 (211-1135) | 3 (0-548) |
Results: Compared to control, 205 proteins (127 upregulated, 78 downregulated) were significantly different at baseline in NOMID, compared to 163 proteins (101 upregulated, and 62 downregulated) in CANDLE. 134 dysregulated proteins (85 upregulated, 49 downregulated) overlapped in NOMID and CANDLE (
Conclusion: Differentially expressed proteins in NOMID and CANDLE are consistent with innate immune cell activation. Distinct endothelial cell signatures in NOMID and CANDLE may provide mechanistic insight into differences in vascular phenotypes. Treatment with anakinra and Baricitinib in NOMID and CANDLE leaves 30% and 13% of the dysregulated proteins unchanged.
Acknowledgments: This work was supported by Intramural Research at
National Institute of Allergy Immunology and Infectious Diseases of National Institutes of Health, Bethesda, Maryland, the Center of Human Immunology and was approved by the IRB.
Disclosure of Interests: None declared