

Background: MAS is a severe complication of rheumatic diseases and occurs most frequently in patients with sJIA. Data from animal models and from observational studies in patients suggest that interferon gamma (IFNy) is a driver of the hyperinflammation and hypercytokinemia observed in MAS.
Objectives: To assess the pharmacokinetics, efficacy, and safety of intravenous (IV) infusions of emapalumab, a fully human anti-IFNγ monoclonal antibody, in patients with MAS in the context of sJIA.
Methods: This ongoing, pilot, open-label, single-arm study (NCT03311854) includes patients with MAS (2016 ACR/EULAR criteria) on a background of confirmed, or high presumption of, sJIA, and with inadequate response to high-dose IV glucocorticoids. Emapalumab is initiated at 6 mg/kg (1 dose) and continued at 3 mg/kg twice weekly for a total of 4 weeks, or less upon achievement of complete response (CR). CR is defined as an absence of MAS clinical signs plus white blood cell and platelet counts above the lower limit of normal, LDH, AST and ALT <1.5 x upper limit of normal, fibrinogen >100 mg/dL, and ferritin decreased by ≥80% or to <2,000 ng/mL.
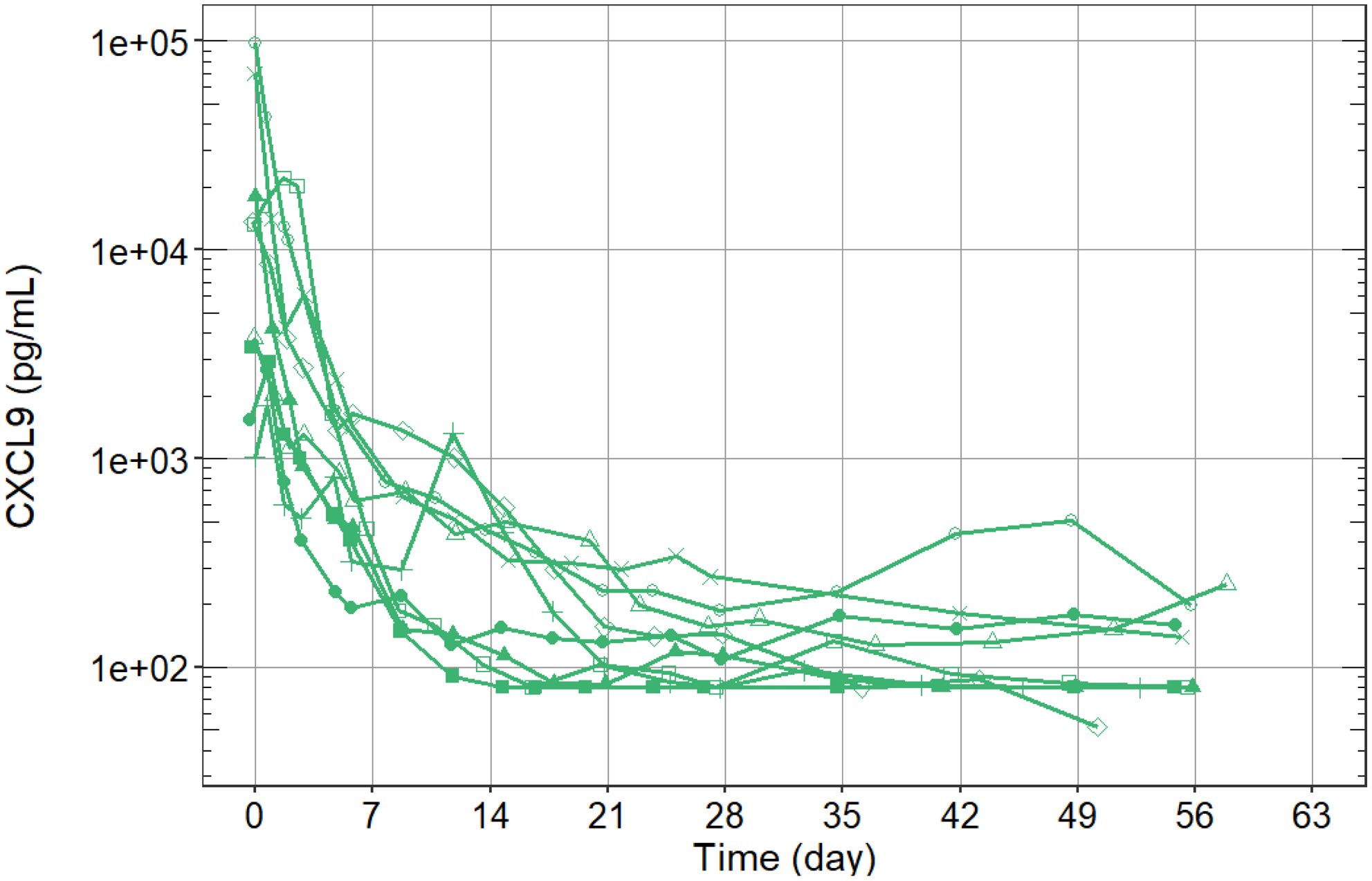
Results: We report preliminary data from the first 9 patients (median age [range] 11.6 [2.1-25.3] years) enrolled (7 in Europe and 2 in the USA). All patients had failed high-dose methylprednisolone, of which there were prior treatment failures from cyclosporin A (n=4) and from anakinra (n=4). Treatment with emapalumab resulted in rapid IFNγ neutralization, as demonstrated by the decrease in CXCL9 levels (
Time to response for key clinical and laboratory parameters.
| Parameters | Median baseline value (range) | Median days of treatment (range) |
|---|---|---|
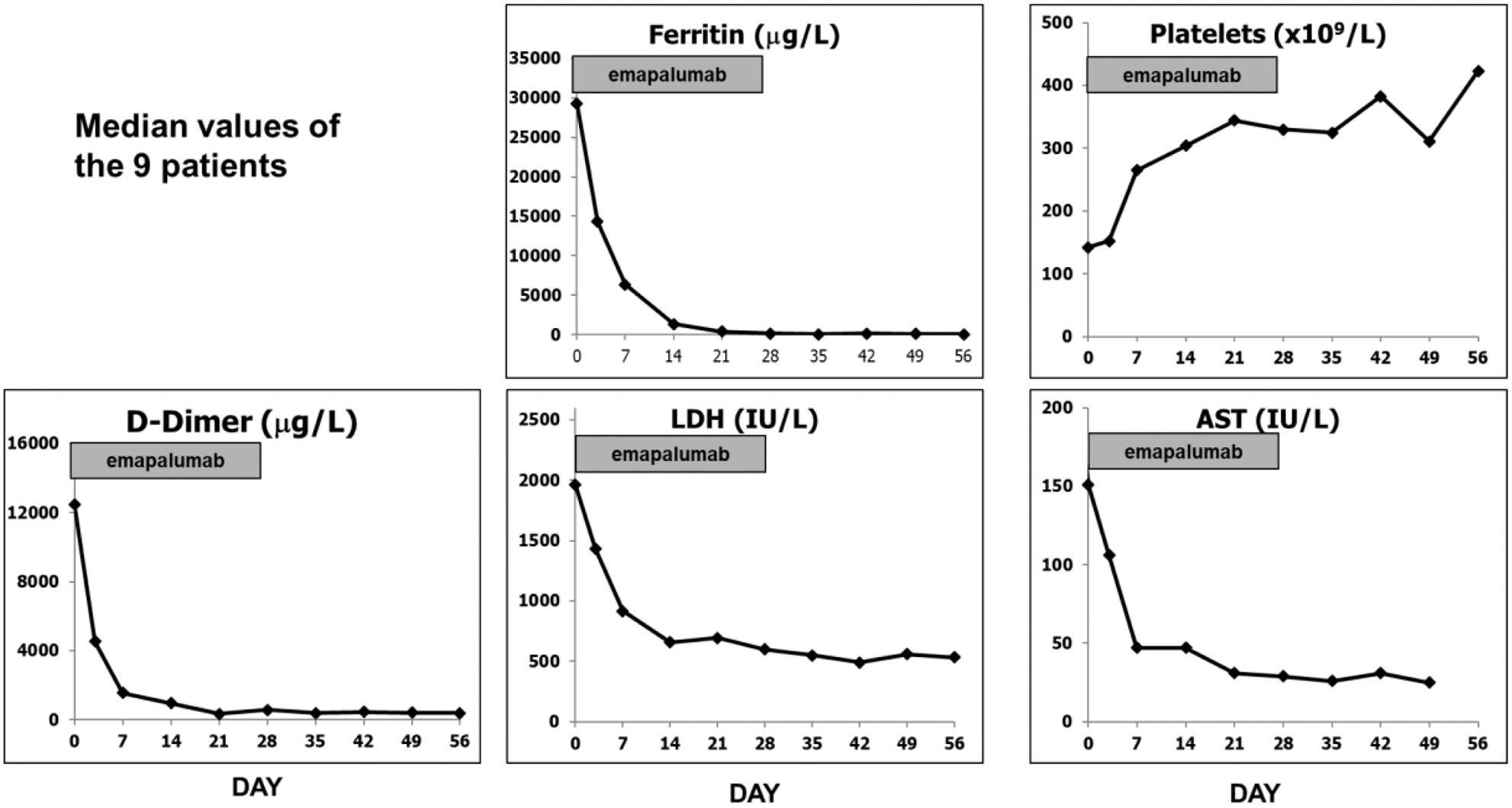
| D-dimers to <1000 mg/L | 12,480 (550-89,552) | 15 (1-49) |
| sIL-2R to <2000 ng/L | 4596 (1664-20,954) | 21 (6-37) |
| Ferritin <500 mg/L | 29,240 (716-192,584) | 21 (9-42) |
| Physician visual analog scale of MAS activity ≤1 | 9.0 (2-10) | 19 (9-56) |
| All MAS laboratory parameters within range of CR | NA | 21 (15-55) |
| All MAS parameters within range of CR | NA | 23 (12-56) |
| Glucocorticoid tapering at ≤1 mg/kg prednisolone equivalent* | NA | 42 (16-50) |
*Data incomplete for 1 patient
Rapid neutralization of IFNy. Each line represents an individual patient (n=9).
Ferritin levels and platelet counts over time.
Conclusion: Emapalumab administration led to rapid neutralization of IFNy and was efficacious in controlling MAS with a favorable safety profile. These results support the pathogenic role of IFNγ in MAS/sJIA and the therapeutic value of IFNγ neutralization in MAS patients who have failed standard of care.
Disclosure of Interests: Fabrizio De Benedetti Grant/research support from: AbbVie, Pfizer, Novartis, Novimmune, Sobi, Sanofi, Roche, Speakers bureau: AbbVie, Novartis, Roche, Sobi, Paul Brogan Grant/research support from: Sobi, Novartis, Roche, Chemocentryx, Consultant of: Roche, Sobi, Speakers bureau: Sobi, Roche, Novartis, UCB, Claudia Bracaglia: None declared, Manuela Pardeo: None declared, Giulia Marucci: None declared, Emanuela Sacco: None declared, Despina Eleftheriou Speakers bureau: Sobi, Charalampia Papadopoulou: None declared, Alexei Grom Grant/research support from: Novartis, AB2Bio, Consultant of: Novartis, Pierre Quartier Consultant of: AbbVie, Chugai-Roche, Lilly, Novartis, Sanofi, Sobi, Speakers bureau: AbbVie, BMS, Chugai-Roche, Novartis, Pfizer, Sobi, Rayfel Schneider Grant/research support from: Roche, Novartis, Sobi, Pfizer, Consultant of: Sobi, Novartis, Novimmune, Philippe Jacqmin Consultant of: Sobi, Rikke Frederiksen Employee of: Sobi, Maria Ballabio Employee of: Sobi, Cristina De Min Employee of: Sobi