

Background: Osteoporosis and renal insufficiency are coexisting disease states in a substantial proportion of postmenopausal women. Since bisphosphonates are generally contraindicated in patients with estimated glomerular filtration rate (eGFR) <35 mL/min, it is important to evaluate other osteoporosis treatments in this setting.
Objectives: To determine if baseline renal function affects the efficacy and safety of romosozumab.
Methods: We performed post hoc analyses of two clinical trials of romosozumab in postmenopausal women with osteoporosis. In ARCH (NCT01631214), 4,093 patients were randomised 1:1 to romosozumab 210 mg monthly or alendronate 70 mg weekly for 12 months (mean age: 74.3 years; 96.1% with prevalent vertebral fractures [VFx]). In FRAME (NCT01575834), 7,180 patients were randomised 1:1 to romosozumab 210 mg or placebo monthly for 12 months (mean age: 70.9 years; 18.3% with prevalent VFx). For these analyses, patients were categorised by baseline eGFR (mL/min/1.73m 2 ): normal renal function (eGFR ≥90), mild renal insufficiency (eGFR 60–89), or moderate renal insufficiency (eGFR 30–59). Least squares mean (LSM) percent change from baseline in bone mineral density (BMD) at the lumbar spine, total hip, and femoral neck; incidence of new VFx and adverse events (AEs); and changes in renal function were assessed for each eGFR category at Month 12 of the double-blind treatment period.
Results: At baseline, most patients had mild/moderate renal insufficiency: 84% in ARCH, 88% in FRAME. In both studies, change from baseline in BMD was significantly higher in the romosozumab group across baseline eGFR categories (Figure). There was an interaction between BMD increase and renal function, and although BMD increase was not as large in women with impaired renal function, differences between romosozumab and control groups remained significant (Figure). In ARCH, among patients with eGFR ≥90, 60–89, and 30–59, the incidence of new VFx (romosozumab vs alendronate) at Month 12 was 3.3% vs 7.3%, 3.2% vs 3.9%, and 3.4% vs 6.2% in ARCH. In FRAME, the incidence of new VFx (romosozumab vs placebo) at Month 12 was 0.5% vs 3.0%, 0.4% vs 1.5%, and 0.6% vs 2.1%.
In both studies, the incidences of AEs and serious AEs were similar in both treatment groups within and across eGFR categories. AEs of mild-to-moderate hypocalcaemia (investigator reported) occurred in two patients in ARCH (one romosozumab [eGFR 60–89] and one alendronate [eGFR ≥90]), and one patient in FRAME (romosozumab [eGFR 60–89]). Five patients in ARCH (all in the alendronate group) and 19 patients in FRAME (14 romosozumab, 5 placebo) had decreases in serum Ca levels (albumin adjusted); in the romosozumab group all were mild (<LLN–8.0 mg/dL) or moderate (<8.0–7.0 mg/dL). A similar percentage of patients in each group had changes in renal function over 12 months of treatment.
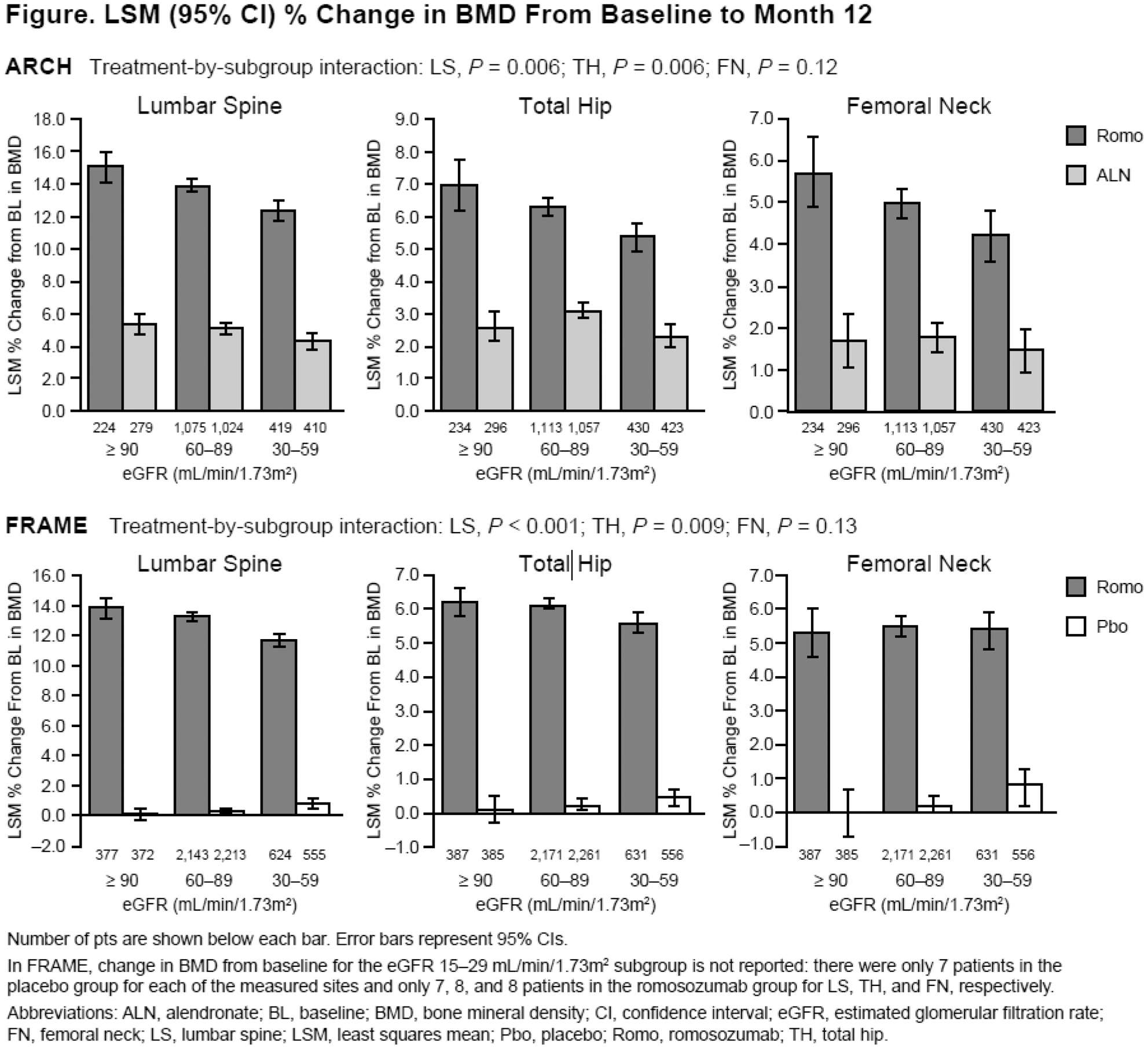
Conclusion: The efficacy and safety of romosozumab vs alendronate or placebo was similar among postmenopausal women with osteoporosis and different levels of renal function.
Acknowledgments: This study was funded by Amgen, Astellas and UCB Pharma. Editorial services were provided by Costello Medical.
Disclosure of Interests: Paul Miller Grant/research support from: Amgen, Radius Health, Ultragenyx, Consultant of: Amgen, Radius Health, Jonathan Adachi Consultant of: Amgen, Speakers bureau: Amgen, Ben-Hur Albergaria Consultant of: Amgen Inc., Eli Lilly, Speakers bureau: Amgen Inc., Eli Lilly, Angela M Cheung Consultant of: Amgen, Eli Lilly, Arkadi Chines Shareholder of: Amgen Inc., Employee of: Amgen Inc., Evelien Gielen Consultant of: Amgen Inc., Takeda, Sandoz and UCB Pharma, Speakers bureau: Amgen Inc., Takeda, Sandoz and UCB Pharma, Bente Langdahl Grant/research support from: Amgen, NovoNordisk, Consultant of: Amgen Inc., Eli Lilly, UCB Pharma, Akimitsu Miyauchi Consultant of: Amgen Inc., Astellas BioPharma K.K., Teijin Pharma, Mary Oates Shareholder of: Amgen Inc., Employee of: Amgen Inc., Ian Reid Consultant of: Amgen Inc., Eli Lilly, Speakers bureau: Amgen Inc., Eli Lilly, Norma Ruiz Santiago Shareholder of: Amgen Inc., Employee of: Amgen Inc., Mark Vanderkelen Employee of: UCB Pharma, Wenjing Yang Shareholder of: Amgen Inc., Employee of: Amgen Inc., Zhigang Yu Shareholder of: Amgen Inc., Employee of: Amgen Inc.