

Background: Personalization of RA treatment is not optimal due to lack of predictors. We previously demonstrated in RA patients that central nervous system (CNS) pain response to tender joint compression, measured by using functional MRI (fMRI) of the brain rapidly wanes after 24 hours of anti-TNF administration and that a higher pre-treatment BOLD signal volume seems to predict clinical response to treatment with certolizumab-pegol (CZP) 1,2 . We therefore hypothesized that the CNS pain response upon compression of a painful joint could predict subsequent anti-TNF treatment response.
Objectives: To compare disease activity after 12-weeks of CZP treatment to that of placebo in DMARD-refractory RA patients based on pre-treatment baseline CNS pain response measured using BOLD fMRI.
Methods: Adult RA patients fulfilling the 2010 ACR/EULAR classification criteria with a DAS28>3.2 under stable DMARD treatment for at least 3 months were eligible. Patients underwent fMRI scanning of the brain at screening for stratification by CNS pain response. Whole brain BOLD-signal-voxel-count of 700 units classifying between low and high, and were randomized to CZP or placebo (2:1) The primary outcome was low disease activity (LDA, DAS28 ≤3.2) after 12 weeks of treatment.
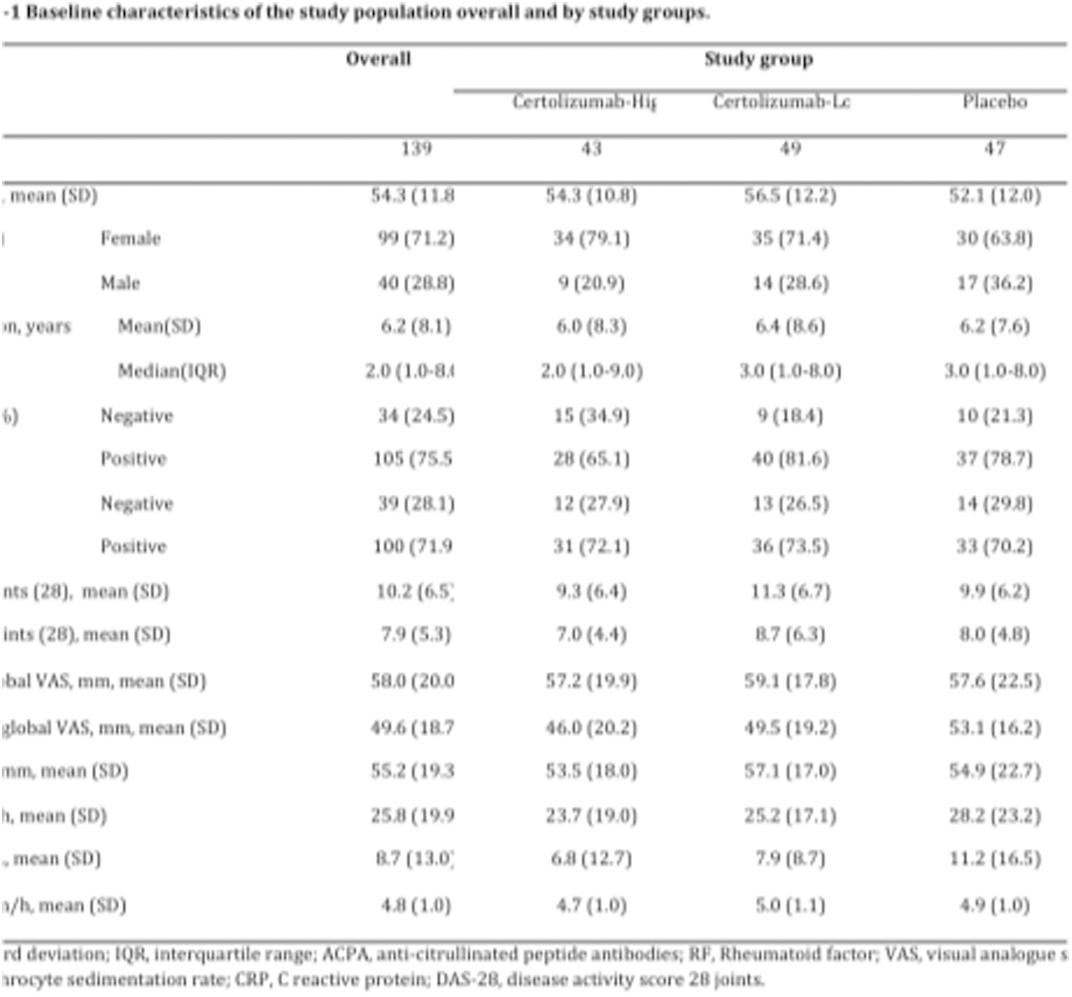
Results: 156 RA patients, inadequate responders to csDMARD, signed the informed consent. 139 patients (46/47, 46/49 and 42/43) (99 females, 71%) with moderate-high disease activity (mean (SD) DAS-28: 4.83 (1.03)) could be included respectively and completed the 12-week study treatment. Geometric mean (SD) numbers of baseline BOLD signal positive voxels were 559 (10), 81 (12) and 2498 (3) in the 3 arms respectively. The mean DAS28 (SD) scores after 12 weeks of study treatment were Placebo: 3.89 (1.29), CZP-L: 3.42 (1.06) and CZP-H: 3.06 (1.04). LDA was achieved in 12/47 patients (25.5 %) in placebo, 22/49 (44.9%) in the CZP-L, and 25/43, (58.1%) in the CZP-H arm. The linear effect term for the ordinal study group variable supported a linear trend of increasing CZP treatment effect with increasing baseline CNS pain response. RR (95% CI) for achieving LDA with each unit increase in treatment category over placebo was 1.79 (1.24 to 2.74, p=0.003).
Conclusion: A higher pre-treatment brain activity in response to pain measured with fMRI predicts the chance of achieving low disease activity with CZP treatment.
REFERENCES:
[1] Hess, A. et al. PNAS (2011)
[2] Rech, J. et al. Arthritis & Rheumatism (2013).
Acknowledgments : The study was supported by an unrestricted grant from UCB Biopharma SPRL, Brussels, Belgium
Disclosure of Interests: Jürgen Rech Consultant of: BMS, Celgene, Novartis, Roche, Chugai, Speakers bureau: AbbVie, Biogen, BMS, Celgene, MSD, Novartis, Roche, Chugai, Pfizer, Lilly, Koray Tascilar: None declared, Hannah Schenker: None declared, Marina Sergeeva: None declared, Mageshwar Selvakumar: None declared, Laura Konerth: None declared, Jutta Prade: None declared, Sandra Strobelt: None declared, Melanie Hagen: None declared, Verena Schönau: None declared, Larissa Valor: None declared, Axel Hueber Grant/research support from: Novartis, Lilly, Pfizer, EIT Health, EU-IMI, DFG, Universität Erlangen (EFI), Consultant of: Abbvie, BMS, Celgene, Gilead, GSK, Lilly, Novartis, Speakers bureau: GSK, Lilly, Novartis, David Simon Grant/research support from: Else Kröner-Memorial Scholarship, Novartis, Consultant of: Novartis, Lilly, Arnd Kleyer Consultant of: Lilly, Gilead, Novartis,Abbvie, Speakers bureau: Novartis, Lilly, Frank Behrens Grant/research support from: Abbvie, Pfizer, Roche, Chugai, Janssen, Consultant of: Abbvie, Pfizer, Roche, Chugai, UCB, BMS, Celgene, MSD, Novartis, Biotest, Janssen, Genzyme, Lilly; Boehringer; Sandoz, Speakers bureau: Abbvie, Pfizer, Roche, Chugai, UCB, BMS, Celgene, MSD, Novartis, Biotest, Janssen, Genzyme, Lilly; Boehringer; Sandoz, José Antonio P. da Silva Grant/research support from: Pfizer, Abbvie, Consultant of: Pfizer, AbbVie, Roche, Lilly, Novartis, Christoph Baerwald Consultant of: CGB received speaker or consulting fees from AbbVie, Paid instructor for: CGB received speaker or consulting fees from AbbVie, Speakers bureau: CGB received speaker or consulting fees from AbbVie, Stephanie Finzel: None declared, Reinhard Voll: None declared, Eugen Feist Consultant of: Novartis, Roche, Sobi, Lilly, Pfizer, Abbvie, BMS, MSD, Sanofi, Speakers bureau: Novartis, Roche, Sobi, Lilly, Pfizer, Abbvie, BMS, MSD, Sanofi, Arnd Doerfler: None declared, Nemanja Damjanov Grant/research support from: from AbbVie, Pfizer, and Roche, Consultant of: AbbVie, Gedeon Richter, Merck, Novartis, Pfizer, and Roche, Speakers bureau: AbbVie, Gedeon Richter, Merck, Novartis, Pfizer, and Roche, Andreas Hess: None declared, Georg Schett Speakers bureau: AbbVie, BMS, Celgene, Janssen, Eli Lilly, Novartis, Roche and UCB