

Background: Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) are recommended as first line treatment for rheumatoid arthritis (RA) patients, but limited information exists on the comparative risk of cancer associated with their use.
Objectives: To compare the risk of incident overall (excluding non-melanoma skin) and site-specific cancers (colorectal, lung, lymphoma, leukaemia) associated with first-line use of csDMARDs in patients with RA.
Methods: We conducted a multinational cohort study informed by data from 7 healthcare databases including claims and electronic medical records from 4 countries (SIDIAP-Spain, MDCR-US Optum-US, CCAE-US, IQVIA AMBEMR-US, IQVIA-Germany, THIN-UK) part of the Observational Health Data Sciences and Informatics (OHDSI) network. All patients aged ≥18 years who initiated methotrexate (MTX), hydroxychloroquine (HCQ), sulphasalazine (SSZ), or leflunomide (LEF) as first-line monotherapy after a diagnosis of RA between 2005 to 2018 were eligible. Individuals with a prior diagnosis of another inflammatory arthropathy or cancer, or <1 year of follow-up were excluded. Patients were followed from 1-year after treatment initiation to the earliest of incident cancer, loss to follow-up, or 5-years. Cox proportional-hazard models for MTX against each other csDMARD were performed after propensity score stratification. A large set of negative control outcomes were analysed to calibrate hazard ratios (cHRs). Estimates were pooled where homogeneity across sources was adequate (I 2 <0.4).
Results: Across the databases, 127,547 RA patients initiating csDMARD therapy were included in the analyses (MTX: 73,996, HCL: 36,381 SSZ: 9,383 LEF: 7,787). The pooled incidence rate of overall cancer for MTX was 22.8 per 1,000 person years. The pooled summary and source-specific estimated cHRs for overall cancer are shown below in
Calibrated hazard ratios (cHRs) of overall cancer risk with their respective confidence intervals (95%CI) by study database. Database estimates not reported where adequate covariate balance not attained. Meta-analysis results not reported where I2>0.4.
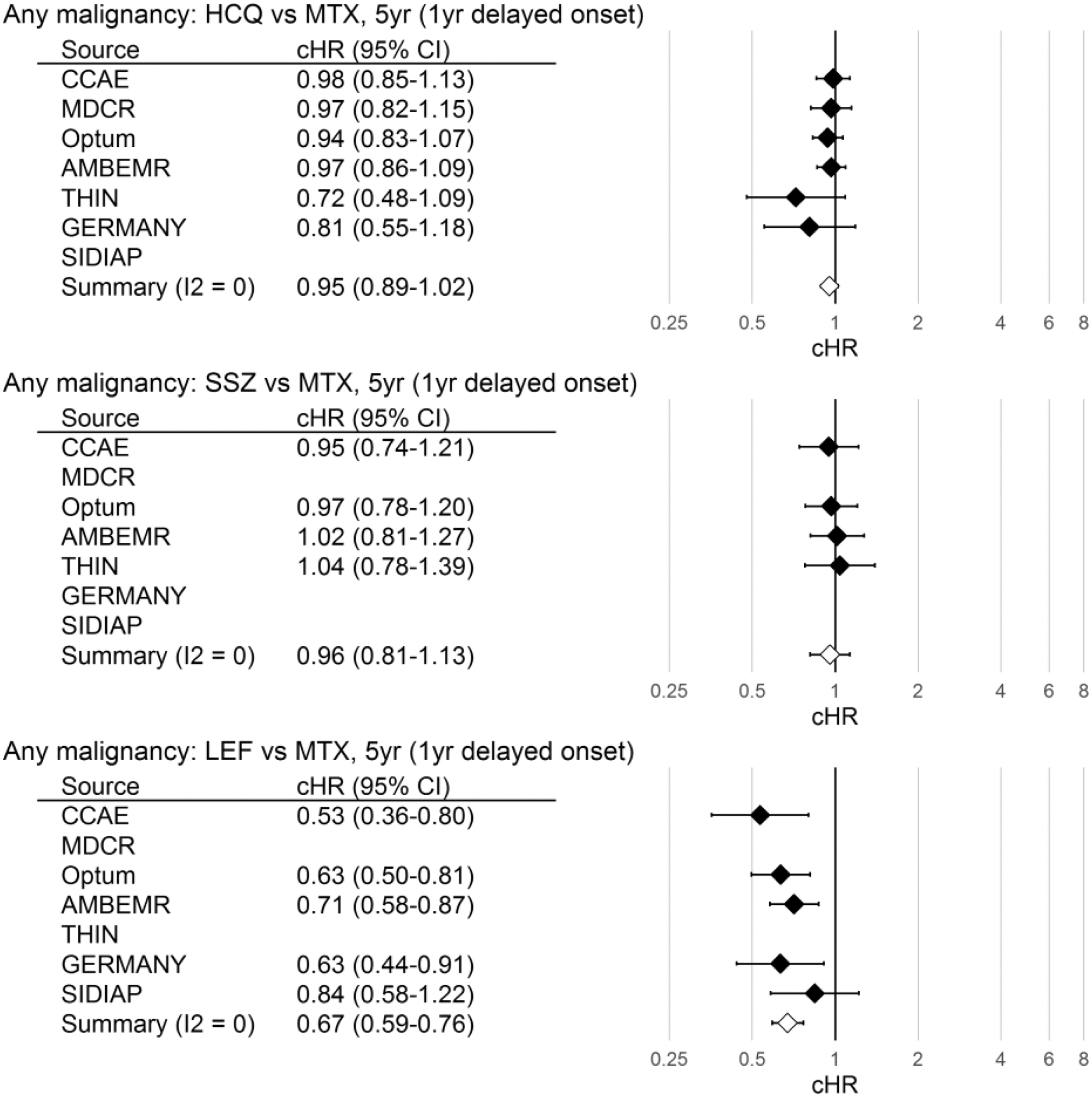
Conclusion: Compared to MTX users, patients treated with LEF had a lower risk of overall cancer. Risk of four specific cancers did not differ by first line csDMARD exposure.
Disclosure of Interests : Talita Duarte-Salles: None declared, Martina Recalde: None declared, James Weaver Shareholder of: J&J Shares, Grant/research support from: Full-time employment salary from Janssen, Consultant of: Janssen employee, Employee of: Janssen, Paid instructor for: Janssen employee, have instructed at conferences, Speakers bureau: Janssen employee, have spoken at conferences, Edward Burn: None declared, Karine Marinier Employee of: Servier, Yesika Díaz: None declared, Ben Illingens: None declared, David Vizcaya Employee of: Bayer, Katerina Chatzidionysiou Consultant of: AbbVie, Pfizer, Lilly., Patrick Ryan: None declared, Daniel Prieto-Alhambra Grant/research support from: Professor Prieto-Alhambra has received research Grants from AMGEN, UCB Biopharma and Les Laboratoires Servier, Consultant of: DPA’s department has received fees for consultancy services from UCB Biopharma, Speakers bureau: DPA’s department has received fees for speaker and advisory board membership services from Amgen