

Background: Cytopenia is a known side-effect of conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs) in rheumatoid arthritis (RA). There is a lack of data on the comparative risk of cytopenia with different csDMARDs.
Objectives: To assess the comparative risk of leukopenia and pancytopenia for the most frequently used first-line csDMARDs: methotrexate (MTX), hydroxychloroquine (HCQ), sulphasalazine (SSZ), and leflunomide (LEF).
Methods: The study used data from 7 databases from 4 countries: CCAE, MDCR, Optum, IQVIA Ambulatory EMR (US); IQVIA THIN IMRD EMR (UK); IQVIA Disease Analyzer EMR (Germany); and SIDIAP (Spain). Cohorts included adult patients with a diagnosis of RA from 2005 to 2019 with at least one-year prior follow-up, no prior inflammatory arthritis, initiaton of first-line csDMARD, and no cytopenia in the preceding 30 days. Participants were followed from one day after treatment initiation to the earliest of event occurrence, treatment discontinuation/switching plus 14 days in the on-treatment analysis, five years in the intent-to-treat (ITT) analysis, or loss to follow-up. MTX was used as reference group. Cox models were fitted with propensity score stratification for observed confounding and negative control outcomes calibration for residual error. Estimates across database were pooled where I 2 <40% was seen.
Results: Overall 166,347 patients were included. Pooled rates of leukopenia and pancytopenia for MTX were 10.9 and 3.2 per 1,000 person years, respectively.
Calibrated hazard ratios (95% CI) vs MTX, on-treatment analysis
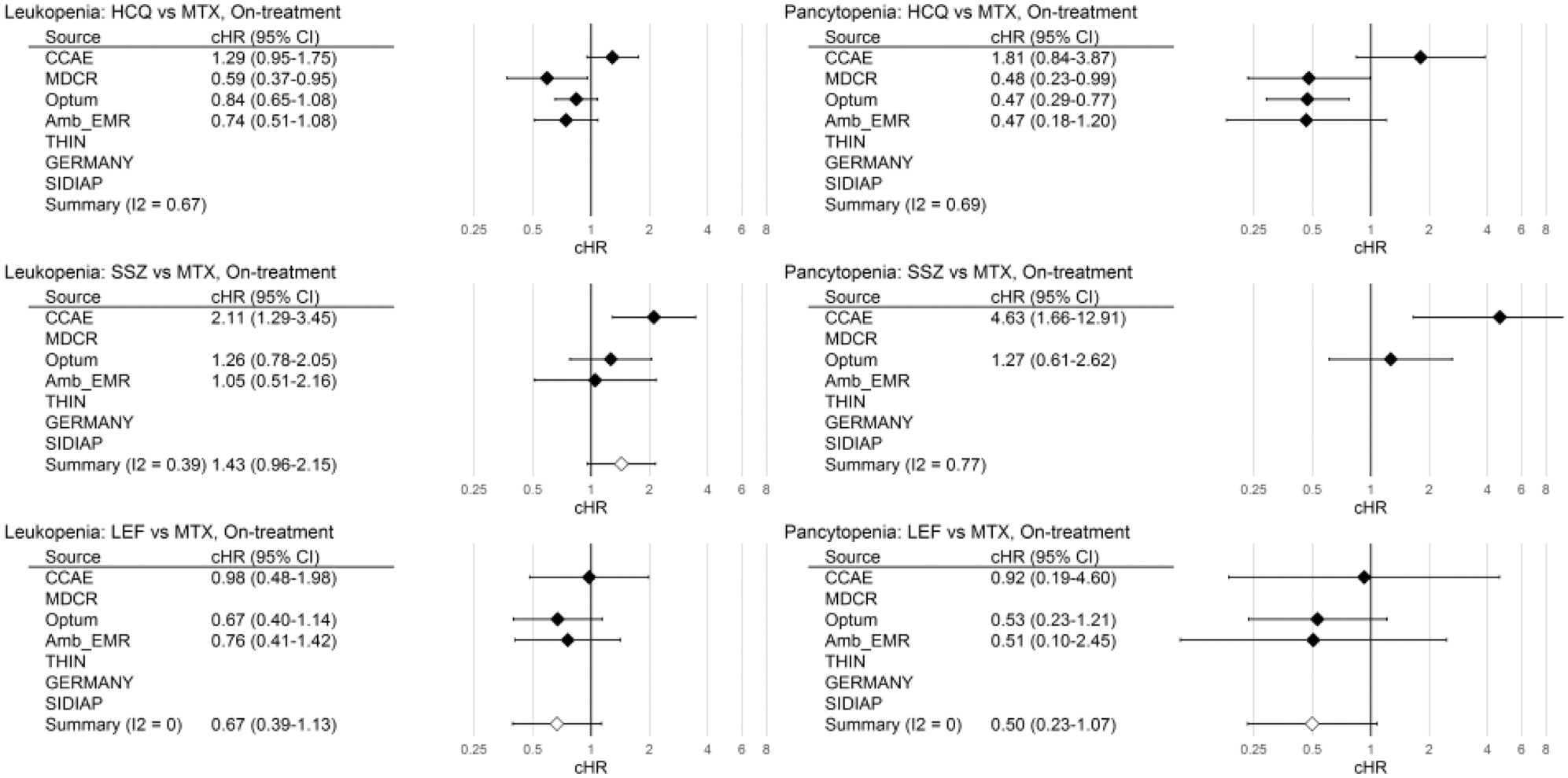
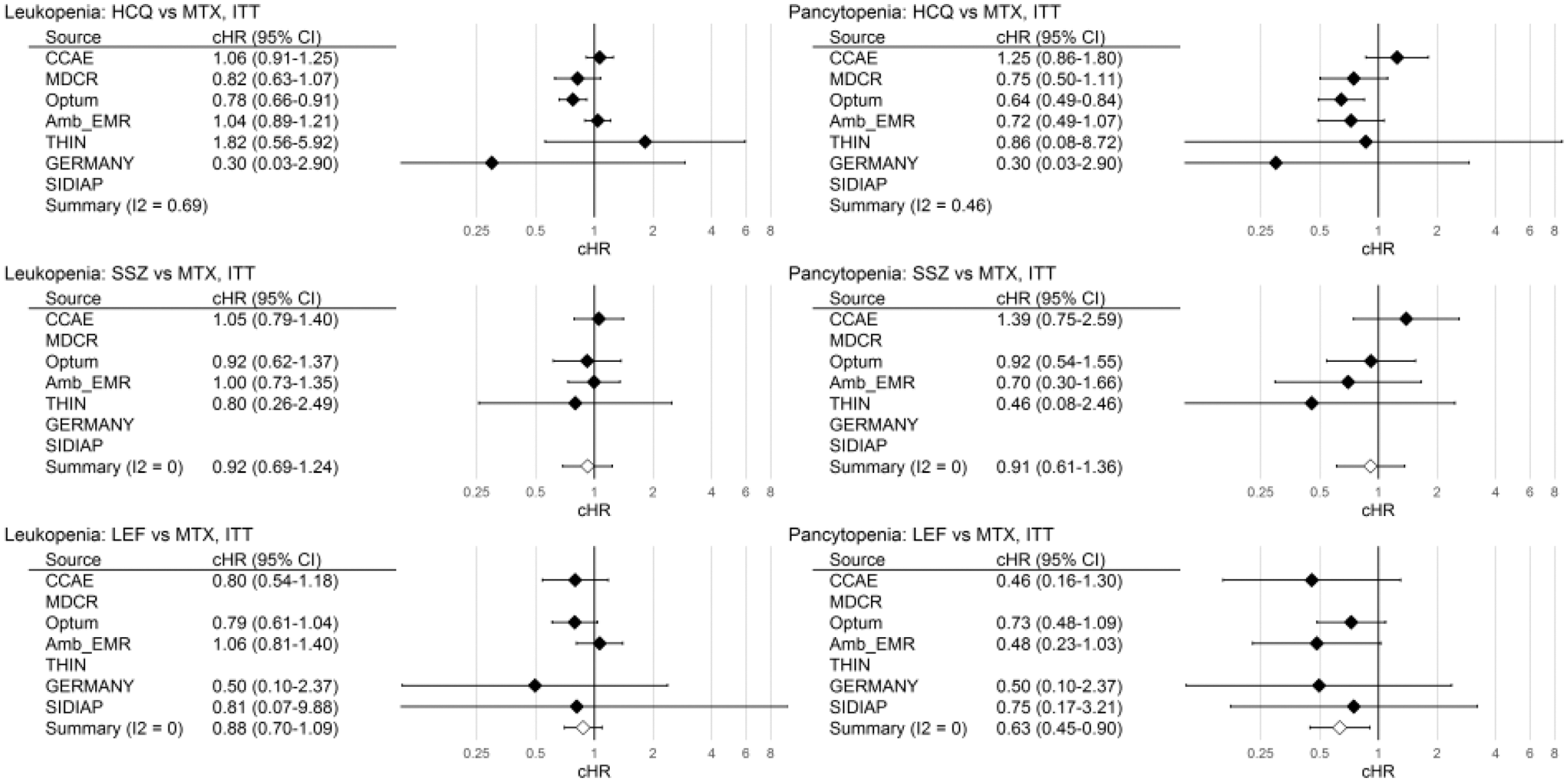
Conclusion: Cytopaenia is rare, and apparently more frequent with MTX and less with LEF. Since prior full blood counts were inconsistently obtained in fewer than 50% of csDMARD new users (e.g. more frequent in MTX [42%] than HCQ [32%] in CCAE and Optum; roughly equal in MDCR), these results should inform future monitoring recommendations.
Calibrated hazard ratios (95% CI) vs MTX, ITT analysis
Disclosure of Interests: Loreto Carmona Grant/research support from: Novartis Farmaceutica, SA, Pfizer, S.L.U., Merck Sharp & Dohme España, S.A., Roche Farma, S.A, Sanofi Aventis, AbbVie Spain, S.L.U., and Laboratorios Gebro Pharma, SA (All trhough institution), James Weaver Shareholder of: J&J Shares, Grant/research support from: Full-time employment salary from Janssen, Consultant of: Janssen employee, Employee of: Janssen, Paid instructor for: Janssen employee, have instructed at conferences, Speakers bureau: Janssen employee, have spoken at conferences, Edward Burn: None declared, Ben Illingens: None declared, David Vizcaya Employee of: Bayer, Ruta Sawant Shareholder of: AbbVie, Employee of: AbbVie, Talita Duarte-Salles: None declared, Patrick Ryan: None declared, Daniel Prieto-Alhambra Grant/research support from: Professor Prieto-Alhambra has received research Grants from AMGEN, UCB Biopharma and Les Laboratoires Servier, Consultant of: DPA’s department has received fees for consultancy services from UCB Biopharma, Speakers bureau: DPA’s department has received fees for speaker and advisory board membership services from Amgen