

Background: Tofacitinib is an oral Janus Kinase inhibitor for the treatment of rheumatoid arthritis (RA).
Objectives: To evaluate the three-year effectiveness of tofacitinib in RA conventional synthetic (cs) DMARDs non-responders.
Methods: Data from 374 patients from Russian national register OREL of patients with RA treated with tofacitinib not less than 3 years after failure of conventional DMARDs were included in the statistical analysis. Clinical and laboratory data from 4 consecutive visits with an interval of 12 months between the visits (± 28 days) were analyzed. Treatment with any biologics ever was an exclusion criteria. Demographical (age, sex) and disease-related characteristics of RA (symptoms duration, RF- and ACCP positivity, presence of joint erosions, DAS28, CDAI, number of tender and swollen joints (NTJ, NSJ), erythrocytes sedimentation rate (ESR), C-reactive protein (CRP)) collected. Statistical analysis performed with statistical programs SPSS2017 and GraphPadPrizm. p-value < 0.05 considered as significant.
Results: Baseline characteristics of RA patients, involved in the analysis are presented in
Baseline characteristics of the patients with RA (n=374).
| Parameter | Characteristics |
|---|---|
| Male, n (%) | 92 (24.5) |
| Age, years (mean±SD) | 53.4±13.38 |
| Symptoms duration, month (mean±SD) | 140±137 |
| Positive rheumatoid factor (RF), n (%) | 123(32.8) |
| Positive antibodies to cyclic citrullinated peptide (ACCP), n (%) | 329(87.9) |
| Erosions of hand joints (X-rays), n (%) | 372 (99.4) |
| BMI, kg/m 2 (mean ±SD) | 26.8 ± 6.14 |
| Smokers (current and in the past), n (%) | 54 (14.4) |
Changes in the diseases activity parameters in patients with RA, treated with tofacitinib not less than 3 years after cs DMARD failure are presented in
DAS28 of patients with RA, treated with tofacitinib (n=374) – 3-years follow-up (time-points are presented in years ± 28 days).
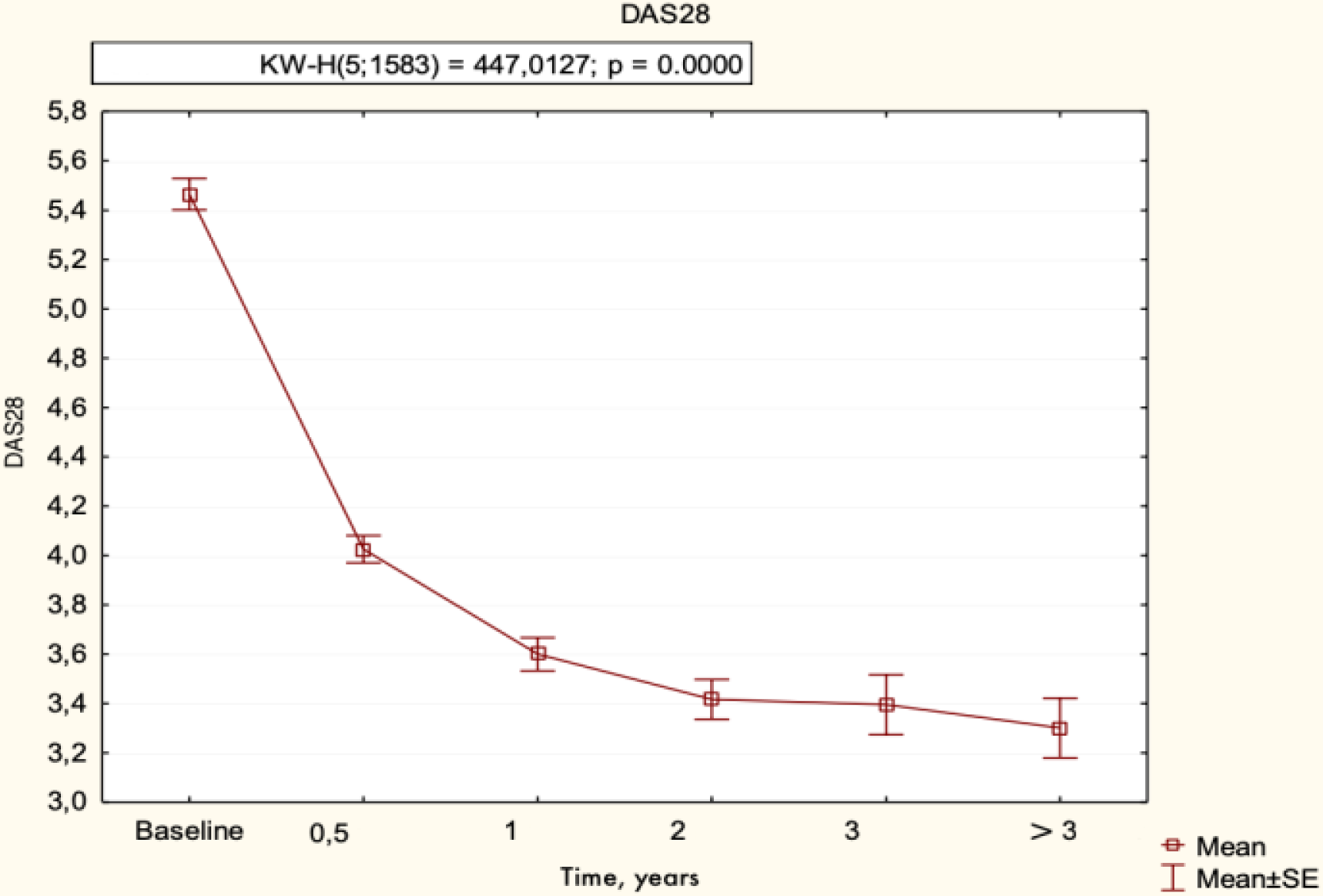
DAS28 of patients with RA, treated with tofacitinib (n=374) – 3-years follow-up (time-points are presented in years ± 28 days).
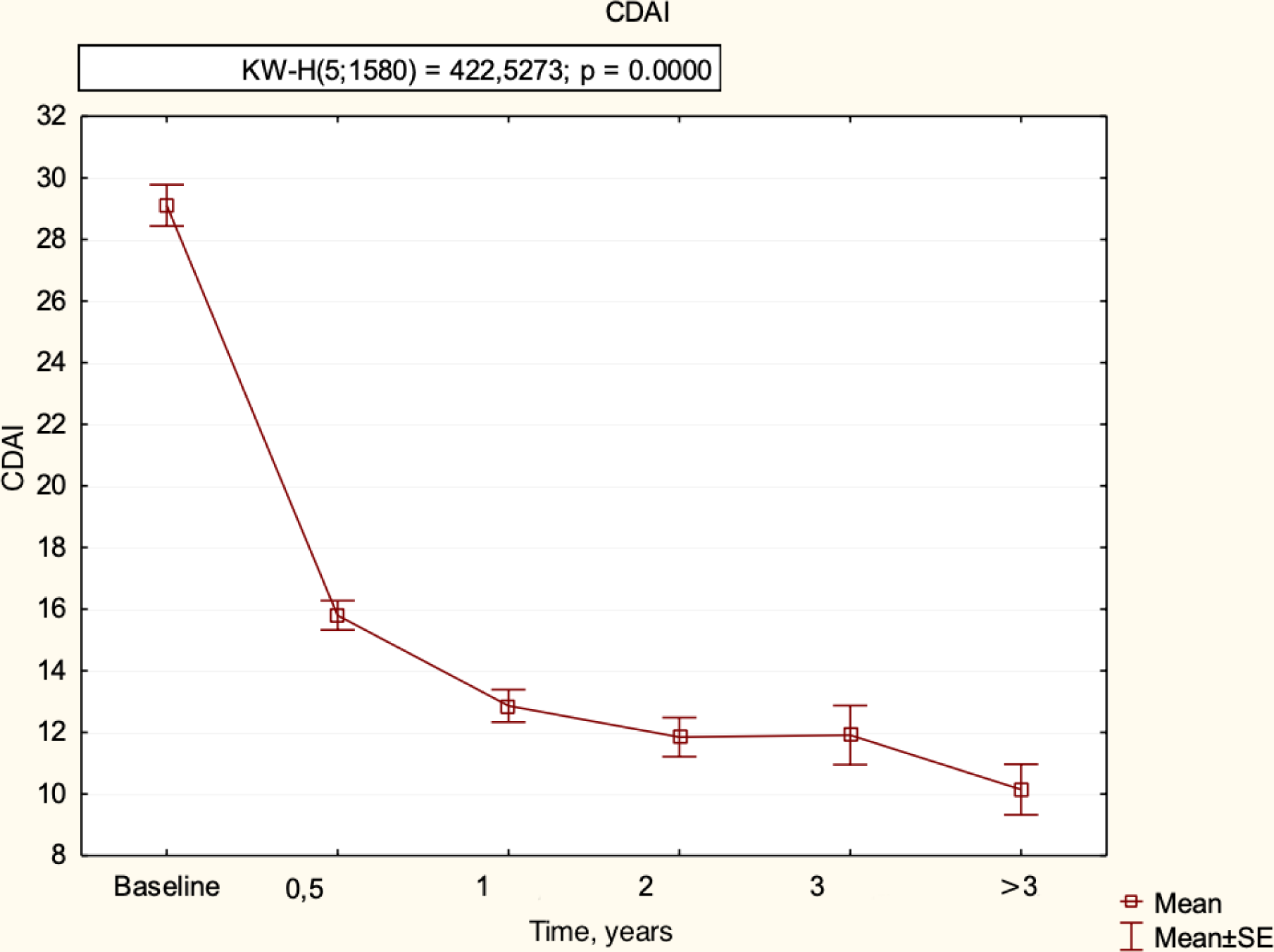
Changes in RA parameters in patients treated with tofacitinib, n=374 (M±SE).
| Disease characteristics | Baseline | Year 1 # | Year 2 # | Year 3 # |
|---|---|---|---|---|
| C-RP, mg/L | 30.1±35.0 | 8.3±12.8 | 7.6±10.7 | 9.4±13.5 |
| ESR, mm/h | 35.2±21.2 | 22.7±17.2 | 21.9±17.7 | 22.3±17.3 |
| NTJ from 28 | 11.2±6.5 | 4.6±4.9 | 4.8±5.0 | 3.9±3.8 |
| NTJ from 28 | 7.6±5.1 | 2.4±3.2 | 1.7±3.1 | 1.4±2.8 |
*difference with baseline is significant with p<0.000. # - ±28 days
Conclusion: According to the real world data treatment with tofacitinib may provide good response rates in RA patients, refractory to the previous csDMARDs treatment in long-term perspective.
Acknowledgments : Pfizer
Disclosure of Interests : Inna Gaydukova Grant/research support from: JSC BIOCAD, Speakers bureau: Pfizer, Novartis, AbbVie, JSC BIOCAD, Сelgene, MSD, Sanofi, V Mazurov: None declared, Alexander Lila: None declared, Andrey Baranov Grant/research support from: Bayer, Galina Lukina Speakers bureau: Novartis, Pfizer, UCB, Abbvie, Biocad, MSD, Roche, Evgeniy Zhilyaev Speakers bureau: Novartis, UCB, Pfizer, Biocad, Abbvie, MSD, Roche, Ekaterina Koltsova: None declared, Evgeniya Shmidt Speakers bureau: MSD, Novartis, Pfizer, Oxana Fomina: None declared, Irina Bondareva: None declared, Olga Anoshenkova: None declared, Aleksey Vasilenko: None declared, Elizaveta Vasilenko: None declared, Natalya Yudina: None declared, Larisa Knyazeva: None declared, Vyacheslav Poncratov: None declared, Ekaterina Gaydukova: None declared, Evgeny Nasonov Speakers bureau: Lilly, AbbVie, Pfizer, Biocad, R-Pharm