

Background: ALPN-101 (ICOSL vIgD-Fc) is an Fc fusion protein of a human inducible T cell costimulatory ligand (ICOSL) variant immunoglobulin domain (vIgD™) designed to inhibit simultaneously the CD28 and ICOS inflammation pathways (1). ALPN-101 is effective in preclinical studies of lupus, arthritis, and Sjögren’s, and shows greater activity than single pathway inhibitors (2,3,4). It is in development for the treatment of multiple rheumatic and other inflammatory diseases.
Objectives: To evaluate the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of ALPN-101 in HV
Methods: This was a first-in-human study of ALPN-101 (NCT03748836). 72 HV were allocated 4:2 to single intravenous (IV) or subcutaneous (SC) doses of ALPN-101: placebo at 0.001 – 10 mg/kg; 24 HV were allocated 6:2 to repeated IV doses of up to 1 mg/kg weekly x 4. Subjects were followed for 28 (SAD) or 49 (MAD) days to assess safety, PK, target saturation (TS) on T cells, circulating cytokines and PD, the latter based on suppression of IgG responses to keyhole limpet hemocyanin (KLH).
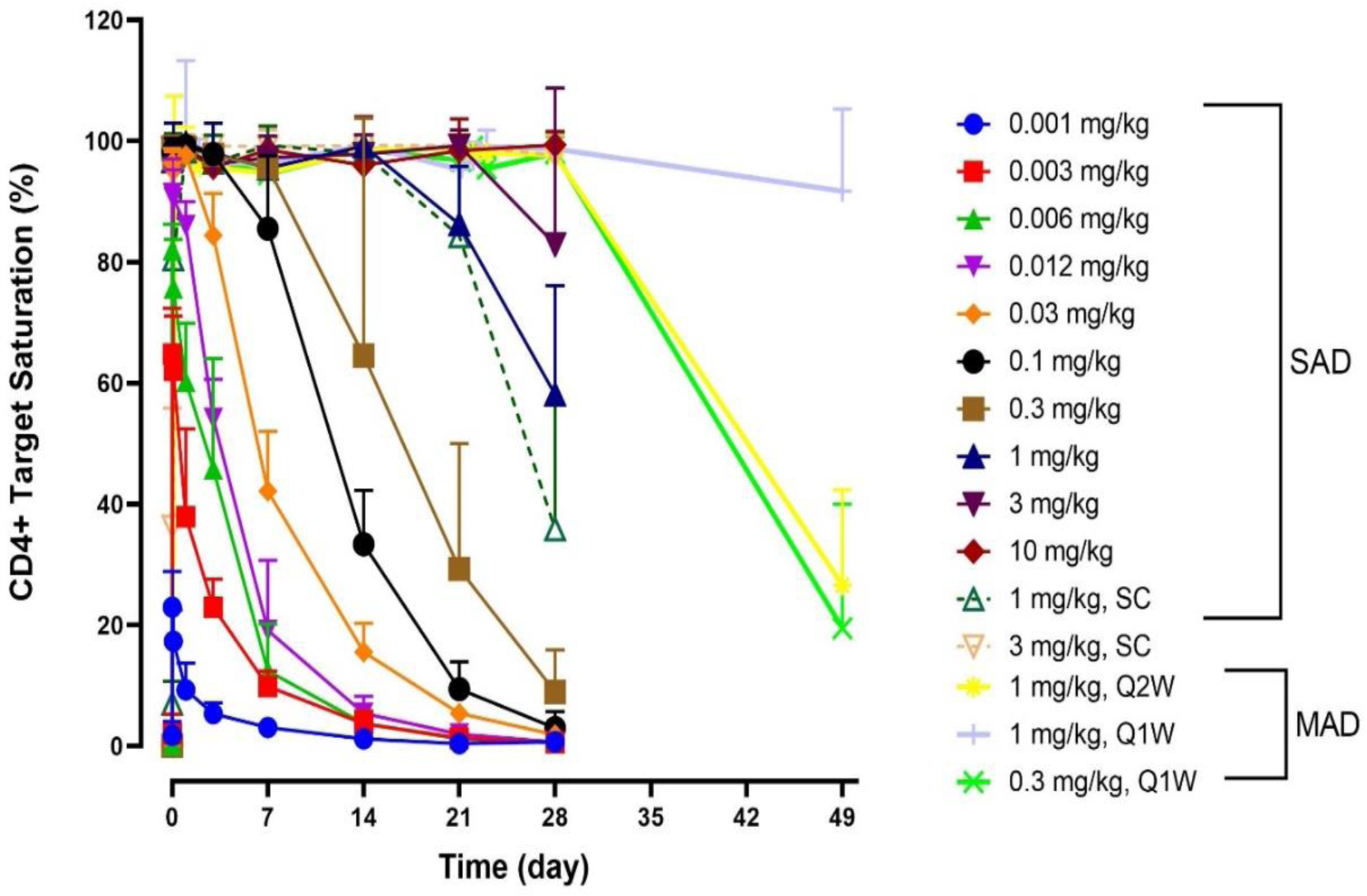
Results: ALPN-101 was generally well-tolerated, with no treatment related serious adverse events, no cytokine release, no clinical immunogenicity, and no adverse trends in safety laboratories. Overall, adverse events were reported in 74.2% of subjects on ALPN-101 and 66.7% of subjects on placebo. All events were mild or moderate and resolved without sequelae. Dose-dependent increase in ALPN-101 exposure was observed from 0.012 to 10 mg/kg. The estimated t
1/2
was 2-8.6 days over 0.3 – 10 mg/kg. SC bioavailability was ~60% at 3 mg/kg. Minimal to modest accumulation was observed with repeat IV dosing. The TS at C
max
increased with dose between 0.001–0.03 mg/kg; thereafter the duration of high level TS (>95%) increased with dose (
Mean + SD Target Saturation of ALPN-101 on Circulating CD4+T Lymphocytes
Mean + SD Serum Anti-KLH IgG Change Relative to Baseline
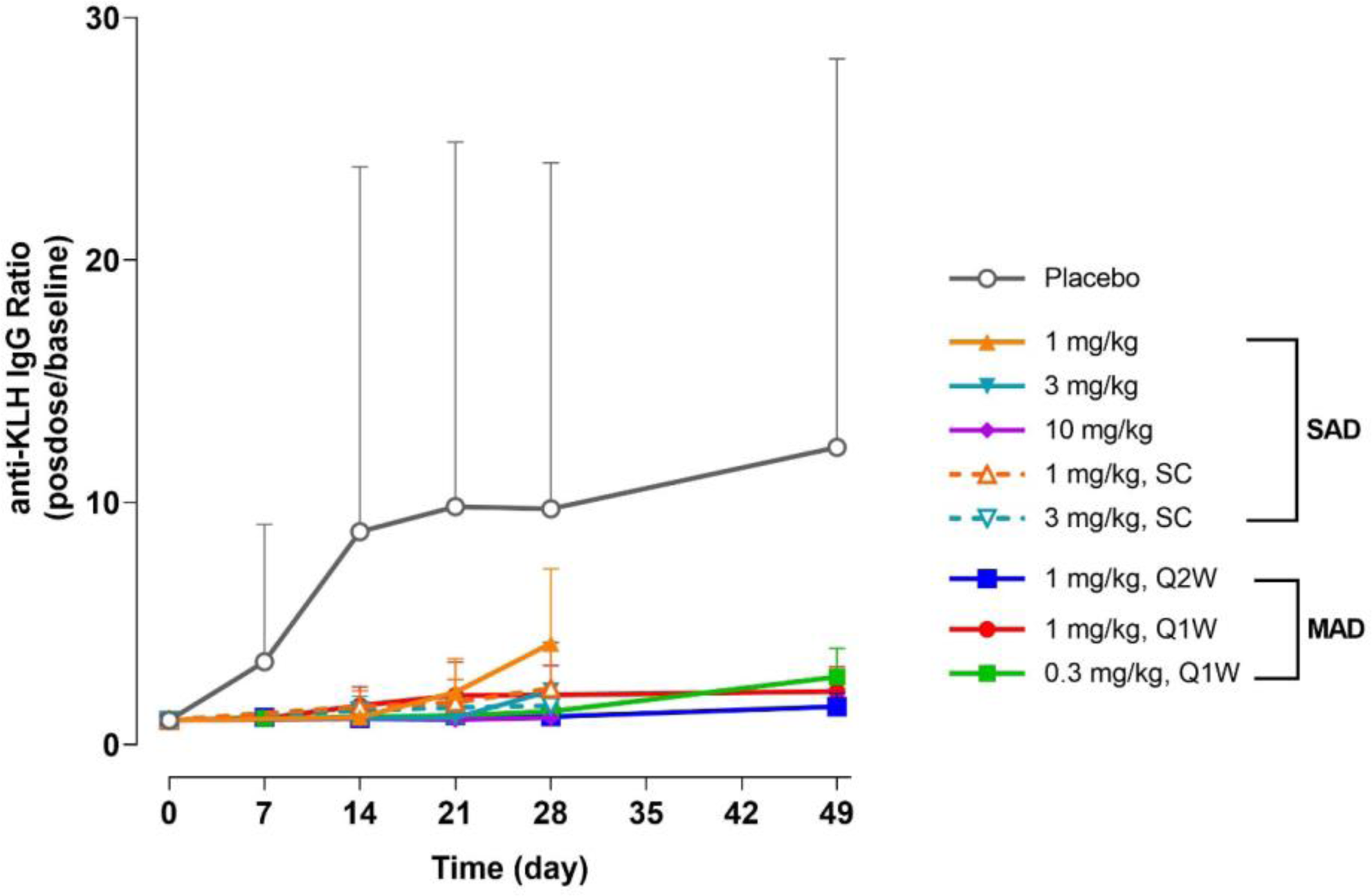
Conclusion: ALPN-101 was well tolerated when administered as single doses up to 10 mg/kg or as repeated doses of up to 1 mg/kg weekly for 4 weeks, exhibiting dose-dependent PK, TS and PD including the inhibition of antibody responses to KLH immunization. These findings support future studies to evaluate the efficacy of ALPN-101 in multiple rheumatic and other inflammatory diseases.
REFERENCES:
[1]Levin SD et al. Frontiers in Immunology 2020; 10:3086
[2]Evans L et al. Arthritis and Rheumatology 2019:71: Supplement: Abstract 1531
[3]Dillon S et al. Arthritis and Rheumatology 2018:70: Supplement: Abstract 136
[4]Dillon S et al. Arthritis and Rheumatology 2019:71: Supplement: Abstract 2416
Disclosure of Interests: Jing Yang Shareholder of: Alpine Immune Sciences, Inc., Employee of: Alpine Immune Sciences, Inc., Jan Hillson Shareholder of: Alpine Immune Sciences, Inc., Employee of: Alpine Immune Sciences, Inc., Jason Lickliter Consultant of: AUD 2500 from QBiotics for participation in an expert review panel for development of their oncology phase 1 trial (in Nov 2015), Kristi Manjarrez Shareholder of: Alpine Immune Sciences, Inc., Employee of: Alpine immune sciences, Inc., Almudena Tercero Shareholder of: Alpine Immune Sciences, Inc., Employee of: Alpine Immune Sciences, Inc., Jennifer Wiley Shareholder of: Alpine Immune Sciences, Inc., Employee of: Alpine Immune Sciences, Inc., Gary Means Shareholder of: Alpine Immune Sciences, Inc., Employee of: Alpine Immune Sciences, Inc., Russell Sanderson Shareholder of: Alpine Immune Sciences, Inc., Employee of: Alpine Immune Sciences, Inc., Kay Carley Shareholder of: Alpine Immune Sciences, Inc., Employee of: Alpine Immune Sciences, Inc., Stanford L. Peng Shareholder of: Alpine Immune Sciences, Inc., Employee of: CMO and President of Alpine Immune Sciences, Inc.