

Background: Sjogren’s syndrome (SS) is a chronic progressive autoimmune disorder which occurs as primary (pSS) or secondary SS (sSS). With no approved disease modifying therapy, there is limited information on the treatment patterns and resource utilization among these patients (pts).
Objectives: To describe pts characteristics, treatment patterns and healthcare resource utilization (HCRU) using electronic health records (EHR) of pts with pSS and sSS treated at the Providence St. Josephs Health system (PSJH).
Methods: Pts ≥18 years of age with at least one clinical encounter with ICD-9-CM or ICD-10-CM diagnosis of SS, between Jan 2013 and Mar 2019 were included. Date of first encounter with SS diagnosis (index date) was used to assess pts demographics. Pt baseline comorbidities were evaluated during the 24 months pre-index period. Treatment patterns and HCRU were assessed during the 12 months post-index follow-up. Descriptive statistics were used to describe pts’ demographic and clinical characteristics, and medications use in the baseline and follow up.
Results: Study cohort included 9,108 SS pts of which 76.5% had sSS diagnosis on index date. Majority of SS pts were women, Caucasian, with mean age of 58.3 yrs, and from western states in the US (
Baseline Demographic and Clinical Pts Characteristics
| SS Pts (n=9,108 ) | |
|---|---|
| Demographics | |
| Age (years) on index date, mean (SD) | 58.3 (15.1) |
| Female, n (%) | 8,338 (91.6) |
| Caucasian, n (%) | 6.936 (76.2) |
| Western Region, n (%) | 8,998 (98.8) |
| Married, n (%) | 5,164 (56.7) |
| Never Smoked, n (%) | 4,847 (53.2) |
| Primary diagnosis, n (%) | 2,137 (23.5) |
| Comorbidities, n (% ) | |
| Cardiovascular | 1,408 (17.2) |
| Endocrine | 3,733 (45.5) |
| Oncology | 800 (9.8) |
| Blood disorders | 1,221 (14.9) |
| Pulmonary | 1,802 (22.0) |
| Neurological | 1,821 (22.2) |
| Liver/Kidney | 1,782 (21.7) |
| Rheumatologic disorders | 2,096 (25.6) |
| Autoimmune/ Immune related | 1,527 (18.6) |
| Baseline Medications, n (% ) | |
| Symptomatic 1 | 1,756 (43.0) |
| NSAIDs 2 | 1,578 (38.6) |
| cDMARDs 3 | 1,435 (35.1) |
| Corticosteroid 4 | 1,393 (34.1) |
| bDMARDs 5 | 266 (6.5) |
1 cevimeline, pilocarpine hydrochloride, ophthalmic insert etc; 2 aspirin, ibuprofen, naproxen; 3 methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, myophenolate mofetil, azathioprine; 4 prednisone; 5 sarilumab, belimumab, ustekinumab, infliximab, adalimumab, certolizumab pegol, golimumab, etanercept, abatacept, tocilizumab, rituximab, tofacitinib, baricitinib
HCRU for pSS and sSS Pts
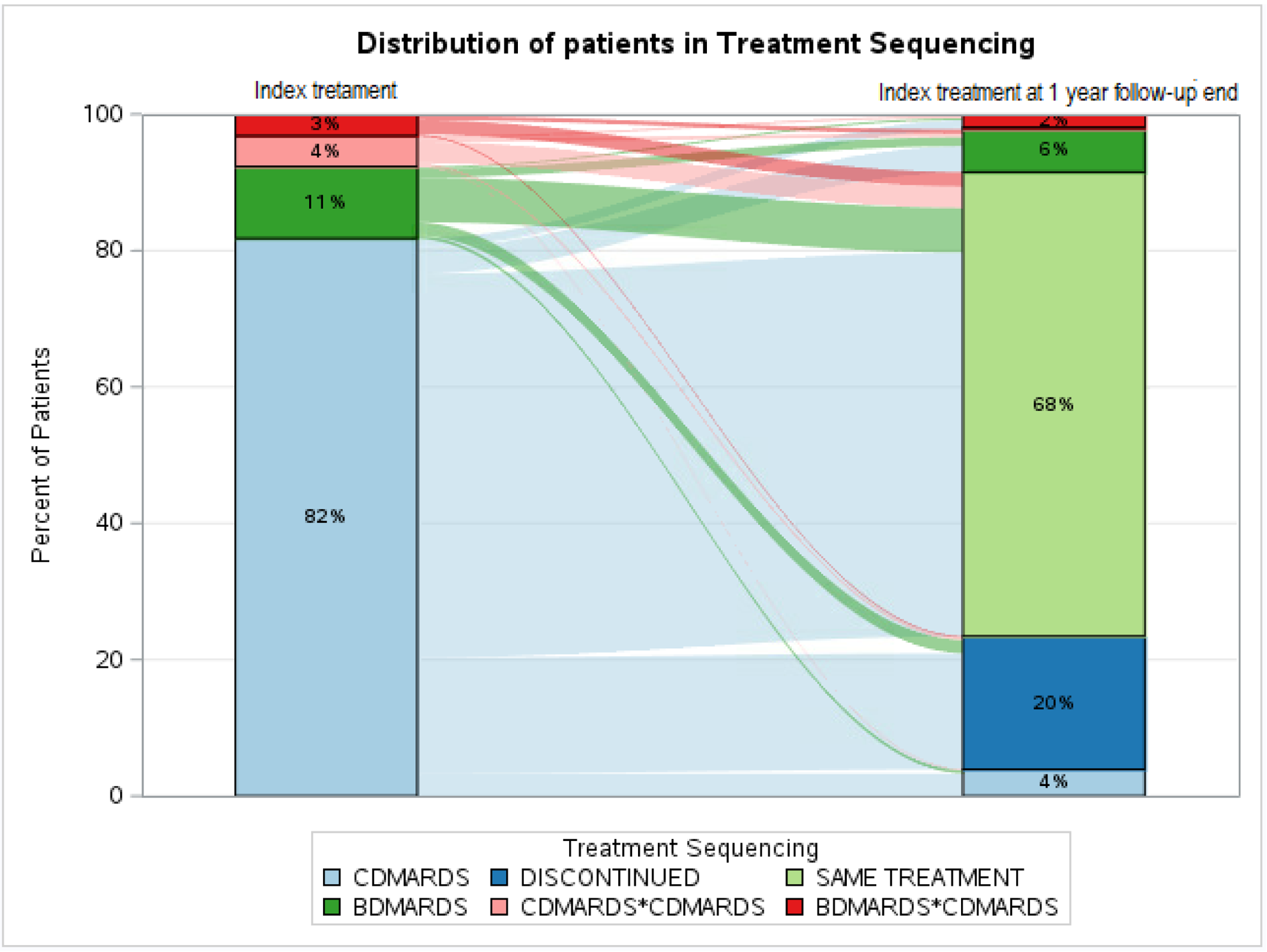
Treatment Sequencing for pSS and sSS Pts. Note: Discontinued: pts who discontinued and didn’t advance to any therapy; same treatment: pts continued on index treatment till we have information.
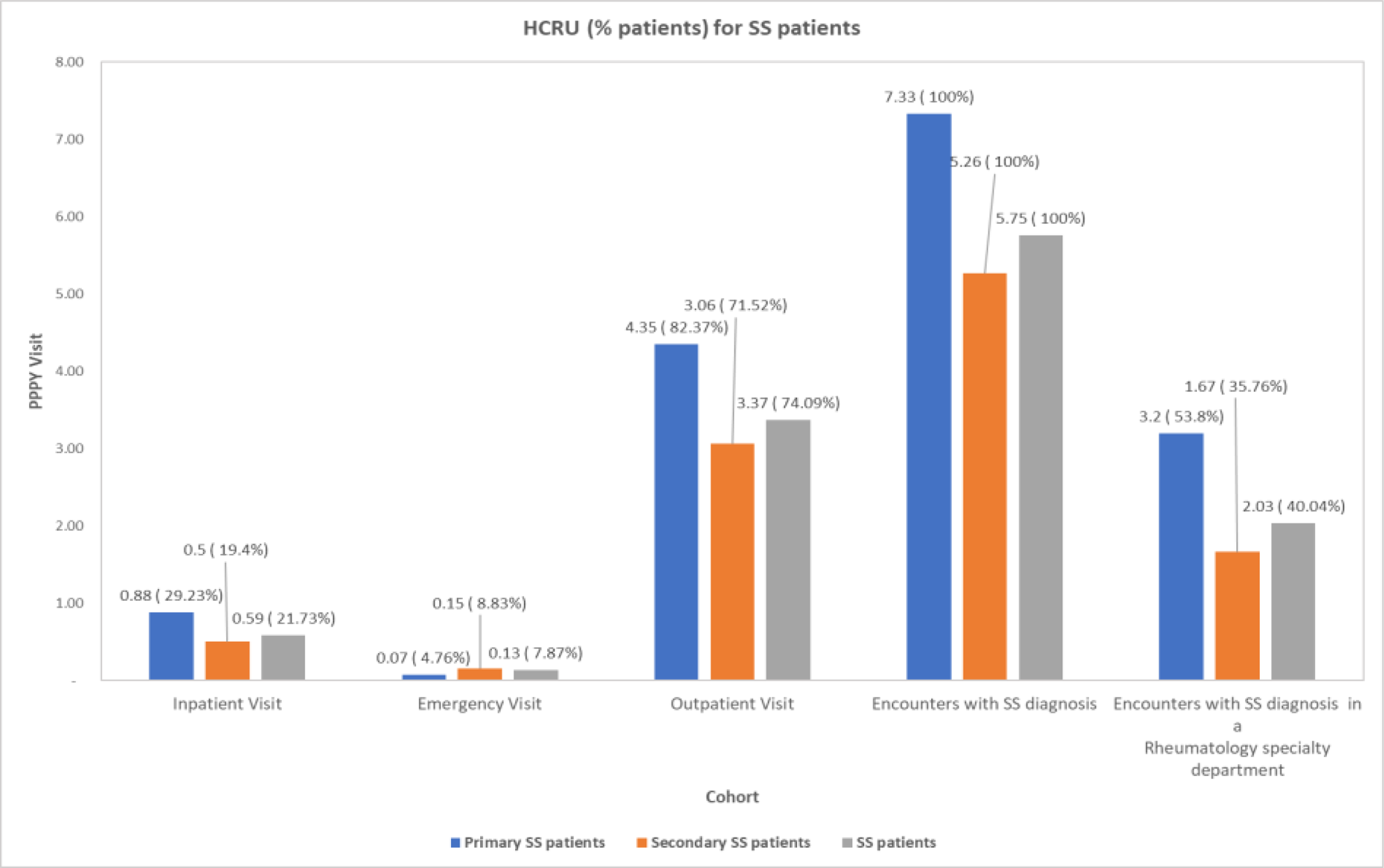
Conclusion: Observation of higher comorbidities suggests substantial burden of SS pts on healthcare system, with majority of pts being diagnosed outside of rheumatology offices.
Acknowledgments : We acknowledge the contributions of Manasi Suryavanshi towards drafting and reviewing the abstract.
Disclosure of Interests: Philip J Mease Grant/research support from: Abbott, Amgen, Biogen Idec, BMS, Celgene Corporation, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, UCB – grant/research support, Consultant of: Abbott, Amgen, Biogen Idec, BMS, Celgene Corporation, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, UCB – consultant, Speakers bureau: Abbott, Amgen, Biogen Idec, BMS, Eli Lilly, Genentech, Janssen, Pfizer, UCB – speakers bureau, Joe Zhuo Shareholder of: Bristol-Myers Squibb, Employee of: Bristol-Myers Squibb, Roshanthi Weerasinghe Grant/research support from:., Qian Xia Shareholder of: I own shares of Bristol-Myers Squibb Company, Employee of: I am a paid employee of Bristol-Myers Squibb Company, Chidananda Samal Consultant of: I work as a consultant for Bristol-Myers Squibb Company, Niyati Sharma Consultant of: I work as a consultant for Bristol-Myers Squibb Company