

Background: Previous studies suggested that male sex may be associated with a greater rate of decline in FVC in patients with SSc-ILD. In the SENSCIS trial, nintedanib reduced the rate of FVC decline over 52 weeks vs placebo.
Objectives: Analyse the rate of decline in FVC and the efficacy and safety of nintedanib in the SENSCIS trial in subgroups by sex.
Methods: Patients with SSc-ILD with first non-Raynaud symptom <7 years before screening and ≥10% fibrosis of the lungs on HRCT were randomised to nintedanib or placebo. We analysed the rate of decline in FVC (mL/year) and adverse events over 52 weeks in male and female patients.
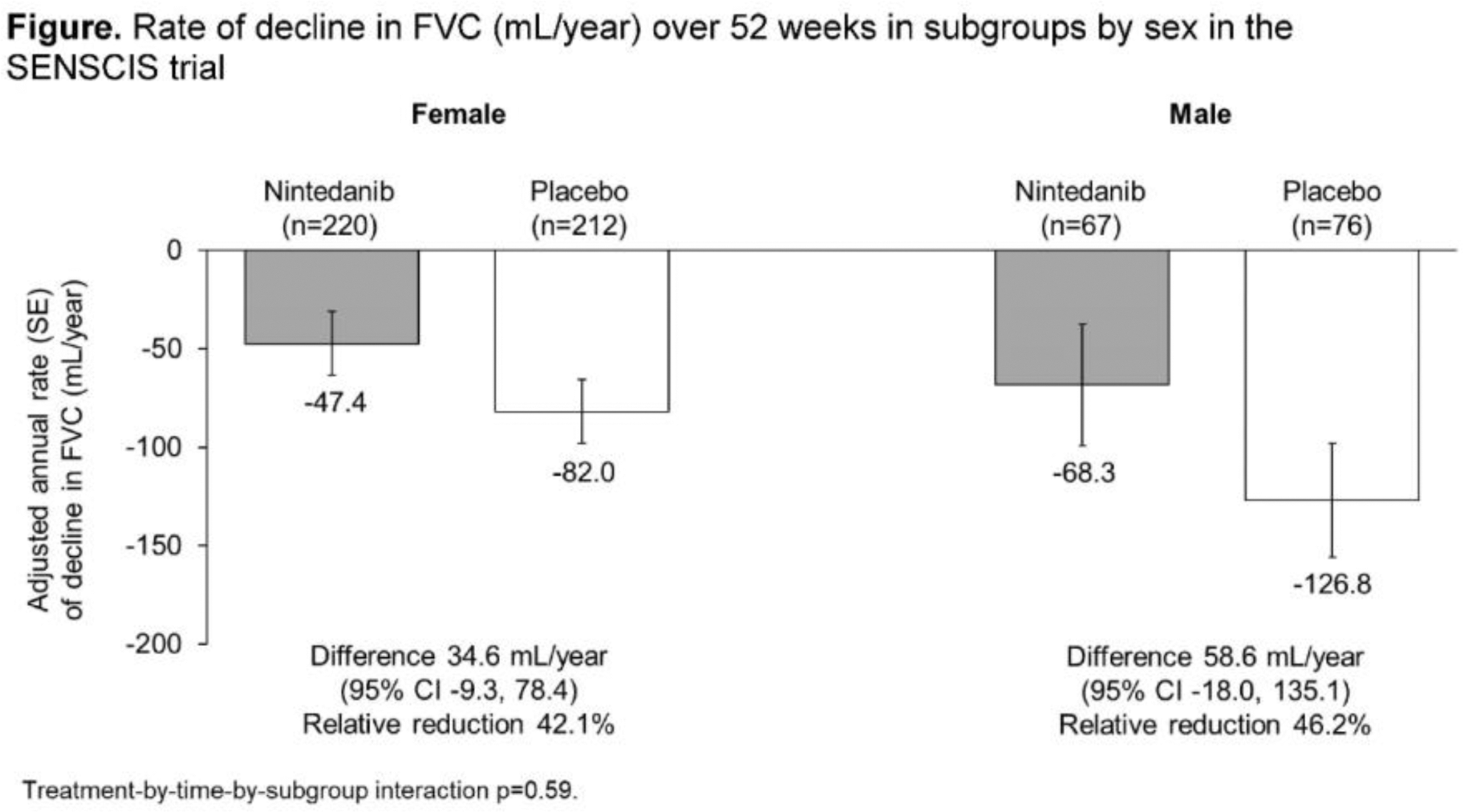
Results: Of 576 patients, 433 (75.2%) were female. Compared with males, the female subgroup included a smaller proportion of White patients (64.7% vs 74.8%), a smaller proportion on mycophenolate at baseline (46.9% vs 53.1%), a greater proportion of ATA positive patients (63.3% vs 53.1%), and had a lower mean weight at baseline (66.6 vs 79.1 kg). FVC % predicted (72.8% vs 71.7%) and mRSS (11.2 vs 10.8) were similar in females and males. The adjusted annual rate of decline in FVC in the placebo group was numerically greater in male than female patients (-126.8 [SE 29.0] vs -82.0 [16.2] mL/year). The estimated effect of nintedanib vs placebo on reducing the rate of decline in FVC was numerically more pronounced in males than females (difference: 58.6 [95% CI -18.0, 135.1] vs 34.6 [-9.3, 78.4] mL/year), but the interaction p-value did not indicate heterogeneity in the treatment effect between subgroups (p=0.59). Among nintedanib-treated patients, diarrhoea was reported in similar proportions of females and males (74.7% vs 79.1%); nausea, vomiting and liver test abnormalities were reported in greater proportions of females vs males (35.3% vs 19.4%, 28.1% vs 13.4%, and 15.4% vs 9.0%), while serious adverse events were more frequent in males (32.8% vs 21.3%). In the nintedanib and placebo groups, respectively, adverse events leading to treatment discontinuation were reported in 16.7% and 8.5% of females and 13.4% and 9.2% of males.
Conclusion: In the SENSCIS trial in patients with SSc-ILD, the annual rate of decline in FVC in the placebo group was numerically greater in male than female patients. The rate of FVC decline was lower with nintedanib than placebo both in males and females. The safety profile of nintedanib was similar between males and females.
Disclosure of Interests: Elizabeth Volkmann Grant/research support from: Forbius, Corbus Pharmaceuticals, Consultant of: Boehringer Ingelheim, Forbius, Speakers bureau: Boehringer Ingelheim, Serena Vettori Consultant of: Boehringer Ingelheim, John Varga Grant/research support from: John Varga is awaiting grants from Boehringer Ingelheim and has received grants from Bristol-Myers Squibb, Pfizer, Takeda, and TeneoBio, Consultant of: John Varga has acted as a consultant for Boehringer Ingelheim, Bristol-Myers Squibb, Emerald Health, and TeneoBio, Ariane Herrick: None declared, Maurizio Cutolo Grant/research support from: Bristol-Myers Squibb, Actelion, Celgene, Consultant of: Bristol-Myers Squibb, Speakers bureau: Sigma-Alpha, Ana Cordeiro Consultant of: Ana Cordeiro has acted as a consultant for Roche, Speakers bureau: Ana Cordeiro has received speaker fees from Boehringer Ingelheim, Lilly, and Vitoria, Valderilio F Azevedo Grant/research support from: Abbvie, Janssen, Bristol-Myers Squibb, Boehringer-Ingelheim, Lilly and Novartis, Consultant of: Lilly, Novartis, Janssen, Boehringer-Ingelheim, Amgen, Pfizer and Abbvie, Speakers bureau: Sandoz, Celltrion, Lilly, Novartis, Janssen, Boehringer-Ingelheim, Amgen, Pfizer and Abbvie, Sindhu Johnson Grant/research support from: Boehringer Ingelheim, Corbus Pharmaceuticals, GlaxoSmithKline, Roche, Merck, Bayer, Consultant of: Boehringer Ingelheim, Ikaria, Christian Stock Employee of: Employee of Boehringer Ingelheim, Martina Gahlemann Employee of: Employee of Boehringer Ingelheim, Lizette Moros Employee of: Lizette Moros is an employee of Boehringer Ingelheim, Margarida Alves Employee of: Employee of Boehringer Ingelheim, Maureen Mayes Grant/research support from: Maureen Mayes has received grants from Boehringer Ingelheim, Corbus, CSL Behring, Eicos, and Galapagos, Consultant of: Maureen Mayes has acted as a consultant for Boehringer Ingelheim, Eicos, and Galapagos. She was a member of the SENSCIS trial Steering Committee (Boehringer Ingelheim)