

Background: Guselkumab (GUS), a monoclonal antibody that specifically binds to the p19-subunit of IL-23, is approved to treat PsO. At Week24 (W24) of the Phase 3, double-blind, PBO-controlled trial in pts with active PsA who were biologic-naïve or prior TNFα inhibitor (TNFi)-treated (DISCOVER-1), GUS 100 mg, given every 4/8 weeks (Q4W/Q8W), demonstrated efficacy for joint & skin symptoms, physical function & quality of life vs PBO; AEs were consistent with GUS safety in PsO.
Objectives: Assess GUS efficacy & safety in PsA through 1 year.
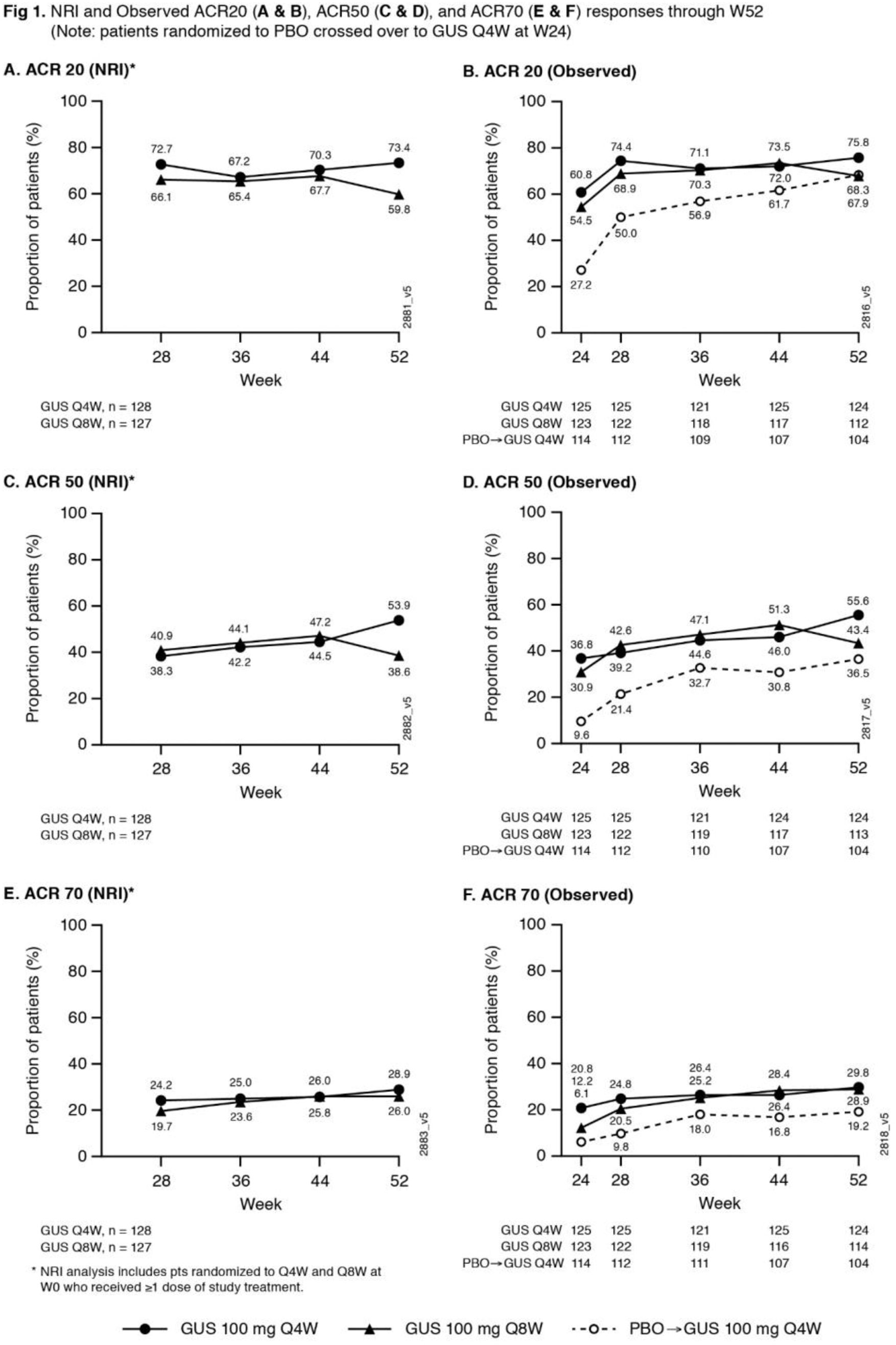
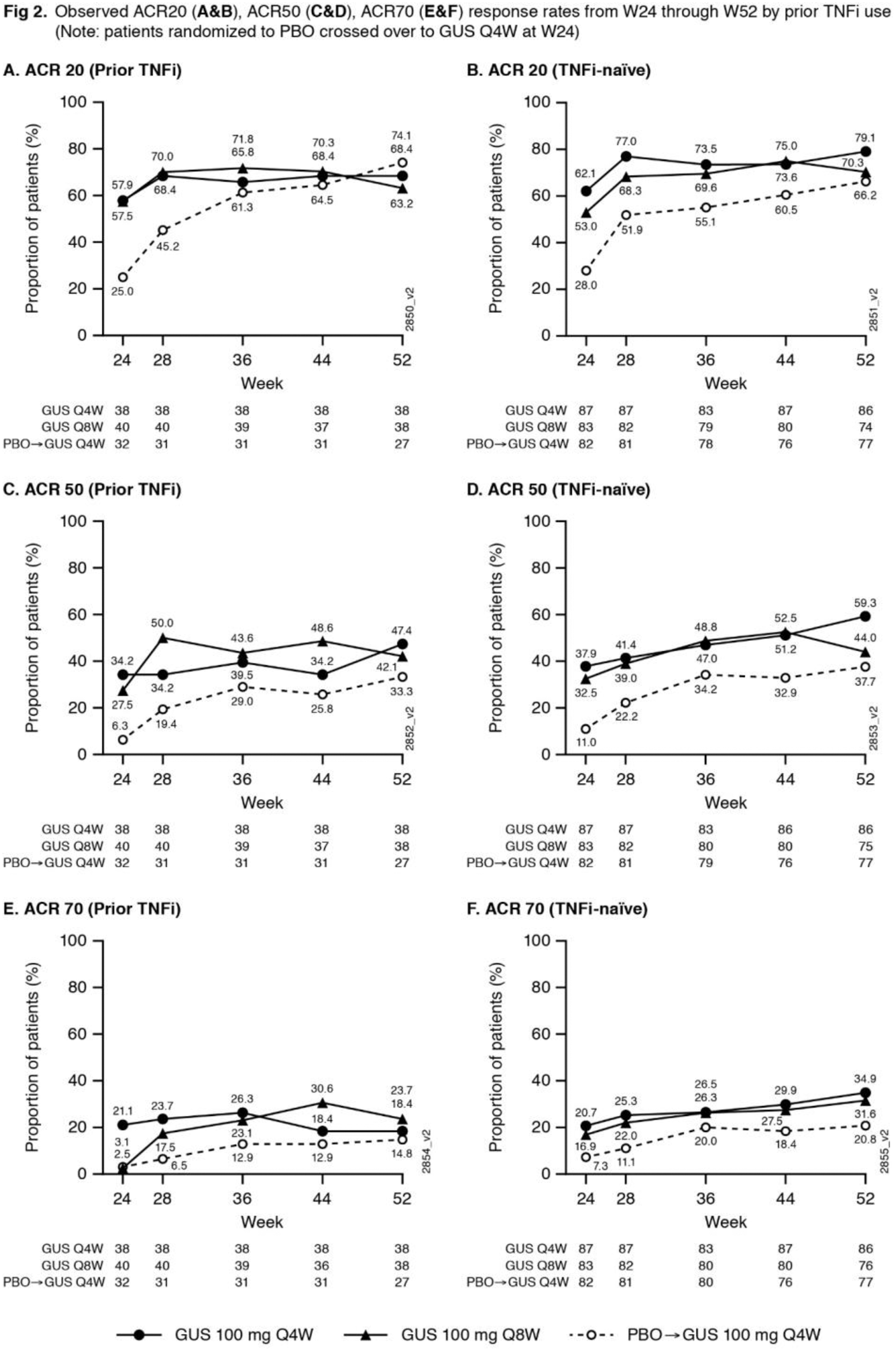
Methods: Adults with active PsA (≥3 swollen+≥3 tender joints; CRP ≥0.3mg/dL) despite standard therapies were eligible. Approx. 30% of pts could have previously received ≤2 TNFi. Pts were randomized 1:1:1, stratified by W0 DMARD [Y/N] & prior TNFi (Y/N) use, to GUS 100mg Q4W; GUS 100 mg at W0, W4 & Q8W; or PBO. At W24, PBO pts crossed over to GUS 100 mg Q4W (PBO X Q4W). W48 marked the last dose of study agent. ACR response rates at W52, based on nonresponder imputation (NRI) for missing data and as observed in pts still on study agent at W24, are shown. Observed data for additional endpoints are shown. AEs through W60 are reported.
Results: 362/381 (95%) randomized pts continued study agent at W24 (125 Q4W, 123 Q8W, 114 PBO X Q4W), 347/381 (91%) pts completed treatment & 343/381 (90%) completed study. NRI ACR20 response rates were maintained at W52 (Q4W 73%, Q8W 60%;
Observed Efficacy 1
| GUS | Q4W | GUS | Q8W |
PBO
|
Q4W
|
|
|---|---|---|---|---|---|---|
| Data are % unless otherwise stated | W24 | W52 | W24 | W52 | W24 | W52 |
| Dactylitis at W0, n | 37 | 37 | 49 | 44 | 47 | 43 |
| Resolution | 64.9 | 78.4 | 67.3 | 79.5 | 61.7 | 81.4 |
| Enthesitis at W0, n | 71 | 70 | 71 | 64 | 71 | 63 |
| Resolution | 49.3 | 62.9 | 40.8 | 56.3 | 31.0 | 69.8 |
| ≥3% BSA psoriasis, IGA ≥2 at W0, n | 88 | 88 | 81 | 75 | 68 | 66 |
| IGA 0/1 + ≥2-grade decrease | 76.1 | 83.0 | 58.0 | 69.3 | 17.6 | 81.5 2 |
| PASI75 | 87.5 | 94.3 | 76.5 | 80.0 | 20.6 | 84.8 |
| PASI90 | 63.6 | 76.1 | 50.6 | 66.7 | 13.2 | 72.7 |
| PASI100 | 45.5 | 64.8 | 25.9 | 48.0 | 7.4 | 62.1 |
| HAQ-DI, n | 125 | 124 | 123 | 114 | 114 | 104 |
| Mean change | -0.4 | -0.5 | -0.3 | -0.4 | -0.1 | -0.4 |
| SF-36 scores, n (mean change) | 124 | 124 | 123 | 114 | 114 | 104 |
| Physical Component - PCS | 6.6 | 8.5 | 6.5 | 7.3 | 2.7 | 6.9 |
| Mental Component - MCS | 3.8 | 4.9 | 3.0 | 5.1 | 1.8 | 4.2 |
| MDA , n | 125 | 124 | 123 | 112 | 114 | 103 |
| MDA response | 31.2 | 40.3 | 23.6 | 33.9 | 12.3 | 31.1 |
| VLDA, n | 125 | 124 | 123 | 114 | 113 | 104 |
| VLDA response | 9.6 | 16.9 | 4.1 | 12.3 | 1.8 | 14.4 |
1 Randomized pts still on study agent at W24; 2 n=65
Conclusion: GUS Q4W & Q8W maintained improvements in joint symptoms through 1 year in pts with active PsA who were biologic-naïve or previously TNFi-treated. In pts continuing in the study, improvements in skin symptoms, dactylitis, enthesitis, physical function & quality of life were also maintained through 1 year. GUS 100 mg Q4W & Q8W were safe and well-tolerated through study completion and consistent with GUS safety in PsO. 1
REFERENCES:
[1]https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TREMFYA-pi.pdf.
Acknowledgments: None
Disclosure of Interests: Christopher T. Ritchlin Grant/research support from: UCB Pharma, AbbVie, Amgen, Consultant of: UCB Pharma, Amgen, AbbVie, Lilly, Pfizer, Novartis, Gilead, Janssen, Philip Helliwell: None declared, Wolf-Henning Boehncke Grant/research support from: Janssen Research & Development, LLC, Consultant of: Janssen, Elizabeth C Hsia Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Ramanand A Subramanian Employee of: Janssen Research & Development, LLC, Xie L Xu Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Shihong Sheng Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Yusang Jiang: None declared, Bei Zhou Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Atul Deodhar Grant/research support from: AbbVie, Eli Lilly, GSK, Novartis, Pfizer, UCB, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myer Squibb (BMS), Eli Lilly, GSK, Janssen, Novartis, Pfizer, UCB, Speakers bureau: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myer Squibb (BMS), Eli Lilly, GSK, Janssen, Novartis, Pfizer, UCB